
Medical equipment batteries have become crucial to the safety, performance, and reliability of many healthcare devices. Modern applications from drones and robotics to medical devices and e-mobility depend on battery technology as their foundation. The healthcare industry sees a steady rise in battery-powered medical devices, and manufacturers must make design choices that will affect their product’s success.
Manufacturers who make poor battery design choices risk overheating, early failure, or regulatory hurdles that can hold up production and shipping. The team behind medical device batteries needs to think about power requirements. They must calculate how much power the battery pack holds, its power delivery speed, and the runtime before recharging becomes necessary. These factors are the foundations of creating rechargeable medical equipment batteries that healthcare environments can rely on.
Companies developing lithium ion batteries for medical equipment need quick market entry. The race to stay competitive makes manufacturers push for faster prototype and production turnaround. But speed cannot compromise the full testing and certification process, which includes UN 38.3, IEC 62133, UL 1642/UL 2054, and CE Marking.
This piece dives into tested methods for designing medical battery packs. We’ll cover everything from core specifications to regulatory compliance. You’ll learn about charge characteristics, mechanical design factors, smart battery features, and safety requirements to help you create reliable power solutions for your medical devices.
Defining Core Battery Specifications for Medical Devices
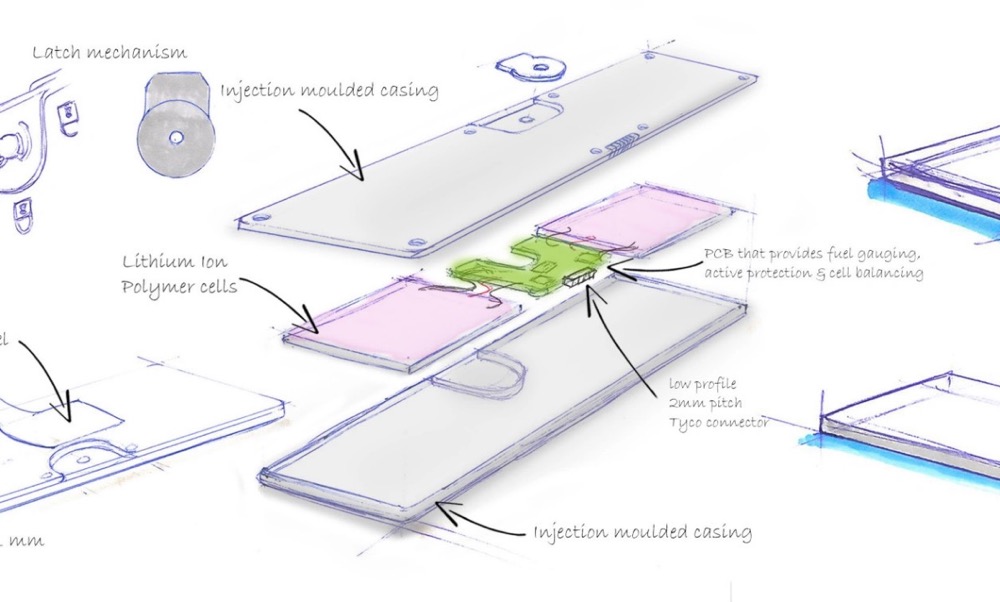
Image Source: Embedded Computing Design
“Primary lithium battery packs are favored for their high energy density and long shelf life, making them ideal for applications requiring reliable power over extended periods.” — Emerging Power Editorial Team, Industry-leading battery solutions provider for medical devices
Battery system design for medical devices starts with choosing the right specifications. A reliable power solution depends on understanding parameters that shape device performance, reliability, and safety.
Nominal Voltage and Capacity Ratings
The nominal voltage shows how a battery performs under normal conditions. This standardized reference value helps predict operational performance. Each battery chemistry comes with its own nominal voltage. LiFePO4 cells provide 3.2V per cell. Standard lithium-ion cells give 3.6V or 3.7V. Primary lithium manganese oxide batteries output 3.0V with a specific energy of 280 Wh/kg.
Battery packs get their nominal voltage by multiplying single cell voltage with series-connected cells. A 48V LiFePO4 battery reaches 51.2V through 16 cells at 3.2V each.
Nominal capacity in ampere-hours (Ah) shows the charge a battery stores and delivers under standard test conditions. This spec determines runtime directly. A 100Ah battery can supply 1A for 100 hours or 10A for 10 hours. Medical devices need detailed documentation of capacity and energy (watt-hours) specs with test conditions.
Shelf Life and Self-Discharge Considerations
Self-discharge substantially affects medical device reliability as batteries lose stored energy even when idle. Primary batteries lose charge slower than rechargeable ones. This makes them great for devices used infrequently. Lithium-ion batteries self-discharge about 5% in the first 24 hours and lose 1-2% monthly after that. Some high-quality lithium metal oxide batteries lose less than 2% charge yearly, lasting up to 20 years.
These factors shape self-discharge:
- Battery chemistry and construction
- Storage temperature (rate doubles every 10°C higher)
- State of charge (higher charges speed up self-discharge)
- Battery age and cycle count
Medical device makers must factor in both cell self-discharge and device current draw to set shelf life limits.
Pack Configuration: Series vs Parallel
Pack configuration shapes voltage, capacity, and reliability heavily. Series-connected cells add voltages but keep capacity constant. Four 3.6V lithium-ion cells in series give 14.4V at the original cell capacity. Parallel connections keep voltage steady but add capacity. These same four cells in parallel deliver 3.6V with four times the capacity.
Series setups provide higher voltages needing less current. This allows thinner wires and reduces voltage drop. Yet one failed cell in series can stop the whole pack.
Parallel arrangements give backup power. The pack keeps working even if one battery fails. This setup suits devices needing longer runtime at lower voltages. Parallel batteries also tend to balance during charge and discharge cycles.
Medical device batteries often use series-parallel combos (like 4s2p) to get both target voltage and capacity. Laptop batteries typically use four 3.6V cells in series for 14.4V. They add two parallel strings to double capacity from 2,400mAh to 4,800mAh.
Charge and Discharge Characteristics in Medical Environments
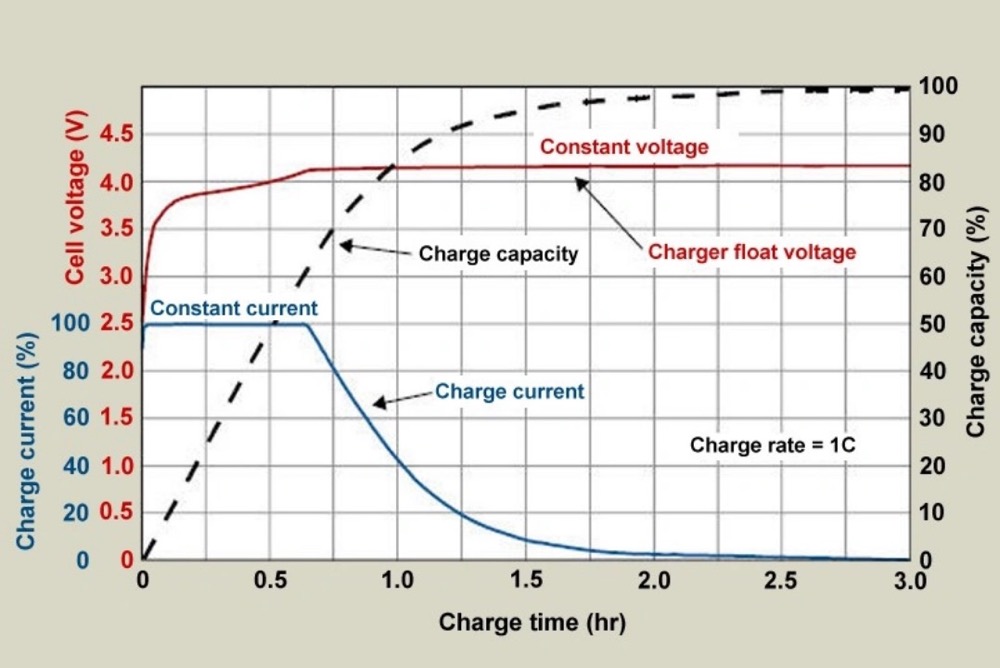
Image Source: Battery University
Battery life and safety in medical equipment depend on the right charging and discharging protocols. Medical device battery makers need to design these protocols carefully. They must work well in healthcare settings where reliable performance can affect patient care.
Constant Current–Constant Voltage (CCCV) Charging
CCCV charging stands out as the safest way to charge lithium-ion batteries in medical devices. This method works in two stages. It starts with constant current until the battery hits its voltage limit, then switches to constant voltage as current drops. Medical applications benefit from this approach because it balances quick charging with longer battery life.
Studies show that CCCV charging lasts three times longer than older methods and cuts charging time by almost 24%. A typical medical-grade lithium-ion battery takes about 50 minutes to charge at 0.5C constant current until it reaches 4.2V. The charging then switches to constant voltage.
Smart CCCV chargers in implantable medical devices can change charging currents based on the battery’s health. A strong CCCV charger improved charging time by 6.5% compared to basic methods by adapting as batteries aged.
Discharge Rate and Runtime Calculations
C-rate shows how fast a battery drains compared to its capacity. A battery discharging at 1C empties in one hour, while 0.5C gives two hours of use. Medical equipment needs accurate runtime predictions to keep patients safe.
You can calculate runtime using this formula: Runtime (hours) = Battery Capacity (Ah) / Discharge Current (A). A 2.5Ah battery running a device that uses 500mA (0.5A) should last about 5 hours. Real-life conditions usually cut this time by 10-20%, so calculations typically use a 0.8-0.9 efficiency factor.
Temperature plays a big role in discharge performance. Cold temperatures slow down the C-rate, while heat speeds it up. Medical equipment works more reliably in temperature-controlled spaces.
Overcharge and Overdischarge Protection
Medical devices need protection systems to prevent dangerous battery failures. Overcharging lithium-ion batteries can damage them forever, reduce capacity, and even cause thermal runaway—where batteries heat up out of control.
A Protection Circuit Module (PCM) keeps batteries safe from overcharging and overdischarging by:
- Watching cell voltages and stopping charge at the safe limit (usually 4.20V per cell)
- Stopping discharge before it goes too low (usually 2.5-3.0V for lithium-ion cells)
- Controlling maximum charge and discharge currents
Overdischarge protection matters most for implanted medical devices. Doctors have reported cases where implanted neurostimulation devices ran out of power completely, which made patients’ symptoms return suddenly. Companies like Medtronic and Boston Scientific now use zero-volt protection technology that helps batteries keep working even after full discharge.
Modern medical batteries use smart protection systems with both electrical and physical safety features. These include special parts that physically break circuits if something goes wrong.
Mechanical Design and Enclosure Considerations
Medical equipment battery enclosures protect and work as functional parts of medical devices. The design must balance protection needs with how the device works while meeting strict healthcare standards.
Ingress Protection Ratings for Medical Use
IP (Ingress Protection) ratings show how well battery enclosures stand up to environmental threats. Medical applications need these specific ratings:
- IP67 rating keeps out dust completely and works underwater up to 1 meter for 30 minutes
- IP68 rating handles deeper water (up to 2 meters) for longer times (1+ hour)
- Medical devices just need IP67 for quick dunks or IP68 when they stay wet longer
Medical equipment goes through tough testing to confirm these ratings. Tests include 8-hour dust exposure and water dunks at specific temperatures (20±5°C). Indoor medical batteries work fine with NEMA 1 rating, but outdoor use needs at least NEMA 3R protection.
Thermal Management in Compact Enclosures
Heat control brings unique challenges to medical battery design. Batteries create heat while running, and this heat needs proper management to avoid early failure or safety risks. Key design elements include:
Lithium polymer cells can grow up to 10% bigger over time, so enclosures need extra space. Vent holes help air flow to cool things down and let gasses escape. Multiple holes in the right spots make air move better.
Powerful batteries need special cooling systems. Some medical device batteries use cooling plates or air conditioning to stay at the right temperature. These cooling systems must stay reliable because if they fail, the battery could overheat.
Shock and Vibration Tolerance for Mobile Devices
Mobile medical devices take constant physical abuse that can hurt battery life. Research shows different batteries handle vibration differently. Cylindrical batteries suffer the most damage under heavy vibration (10-2000 Hz), losing power capacity and getting more internal resistance. Pouch batteries handle vibration best.
Battery enclosures should handle shock and vibration forces from normal use and shipping. Tests follow IEC and ASTM standards. 3D-printed enclosures show good results by keeping their shape with tiny frequency changes (within 0.5 Hz) under stress.
Smart Battery Features and User Interfaces

Image Source: Amazon.com
Smart battery systems reshape the scene by turning regular power sources into smart components that can monitor themselves and communicate. These features play a vital role in critical care devices where reliable power directly affects patient outcomes.
Gas Gage Electronics for State of Charge Monitoring
Medical equipment batteries today use advanced fuel gages that track battery capacity with amazing precision. Simple voltage measurements become unreliable with lithium-ion’s flat discharge curve. However, advanced gas gages deliver ±1% accuracy. These systems track the state-of-charge (SoC) by using coulomb counting. This method measures current flowing in and out of the battery.
High-quality medical devices’ fuel gages give several benefits:
- Smart algorithms that track exact charge levels
- Predictions for charging and discharging times
- Quick setup without long calibration
Cell Balancing and Temperature Sensors
Cell balancing plays a significant role in multi-cell medical device batteries. Individual cells develop different characteristics through repeated charge and discharge cycles. This happens because of varying self-discharge rates and leakage currents. These imbalances can reduce pack performance and create safety risks if left uncorrected.
We can balance cells in two ways: passive balancing (“resistor bleeding”) and active balancing (charge shuttling). Active balancing moves energy between cells instead of wasting it. This is a big deal as it means that efficiency improves. Quick active balancing can fix a 2% capacity imbalance in a 2200-mAh cell in just one or two charge cycles.
Temperature monitoring works hand in hand with balancing circuits. Ring terminal temperature sensors that are accurate to ±0.2°C help protect against overcharging and optimize performance.
Battery Indicators and End-of-Life Alerts
Medical applications need reliable end-of-life indicators. Modern systems include vibration alerts that tell users when batteries are running low. Doctors can also check battery performance remotely without seeing patients.
Smart systems can spot unusual battery behavior, which makes the low risk of sudden battery failure even lower. These batteries can show remaining runtime in minutes instead of percentages. The display updates based on how quickly the battery drains.
Ensuring Safety and Regulatory Compliance

Image Source: UL Solutions
“When engineers develop lithium batteries for medical applications, they must comply with standards set by IEC 60086-4 to ensure the batteries’ electrical, mechanical and chemical safety.” — Large Power Team, medical battery pack manufacturer
Safety shapes every aspect of medical battery design. Regulations establish strict standards that power systems in healthcare must follow.
Internal Short Circuit Mitigation Strategies
Internal short circuits create major risks and can trigger thermal runaway in lithium-ion batteries. Manufacturers use Protection Circuit Modules (PCM) to prevent these failures. These modules control maximum charge/discharge voltages and currents. The system automatically switches to open circuit mode when it exceeds thresholds. Even tiny manufacturing flaws like microscopic metal particles can create internal shorts. Quality control during production remains crucial.
Battery Safety Standards: UL, IEC, UN
Medical battery packs need to meet many standards. The FDA accepts UL 1642 (Lithium Batteries) and UL 2054 (Household and Commercial Batteries) as key standards for medical devices. IEC 62133 lists requirements for secondary cells during normal use and expected misuse. UN 38.3 certification demands eight tests to ensure transportation safety. These tests check altitude simulation, thermal testing, vibration, shock, and short circuit performance. Batteries must pass these tests to prove they can handle shipping and operation conditions.
Labeling, Traceability, and Disposal Guidelines
Battery labels must show type, voltage, capacity details, and safety warnings. Medical device makers need written Standard Operating Procedures to track devices throughout distribution. Batteries don’t belong in regular trash because they pose fire risks. Battery terminals need tape or individual bags before recycling to stop dangerous short circuits.
Conclusion
Medical battery pack design is a vital element in the changing digital world of healthcare technology. This piece explores everything manufacturers must think over when they develop power solutions for medical devices. A battery’s selection affects device performance, reliability, and ended up influencing patient safety in critical healthcare environments.
Battery specifications are the foundations of working medical power systems. Device runtime and operational capabilities depend on voltage, capacity, self-discharge rates, and pack configurations. These parameters need careful optimization based on the device’s specific requirements and how it’s used.
Battery longevity and safety heavily rely on charge and discharge characteristics. CCCV charging protocols, discharge rate calculations, and complete protection mechanisms ensure reliable performance and prevent dangerous failures. Medical device manufacturers should address these factors really well during development.
Mechanical design goes beyond simple containment. The enclosures protect the battery physically, control thermal conditions, and fight environmental threats through appropriate IP ratings. On top of that, it’s particularly important for portable medical equipment to tolerate vibration since it faces constant movement and handling.
Smart battery systems change basic power sources into intelligent components that can monitor and communicate. Gas gage electronics, cell balancing, temperature sensors, and accessible interfaces let users manage battery conditions proactively – a vital capability for critical care devices.
Safety stays at the forefront of medical battery systems. Internal short circuit prevention, following UL/IEC/UN standards, and proper labeling ensure operational safety and regulatory acceptance. These requirements aren’t just bureaucratic hurdles – they’re vital safeguards for patient well-being.
Creating effective battery packs for medical devices means balancing many competing factors – energy density, safety, reliability, size, weight, and cost. Manufacturers who carefully address these elements can create power solutions that meet both regulatory requirements and clinical needs. Battery technology will without doubt keep advancing, offering new possibilities for medical device manufacturers while requiring continued attention to design, testing, and implementation.
FAQs
Q1. What are the key factors to consider when designing battery packs for medical devices? The main factors include defining core specifications like voltage and capacity, optimizing charge and discharge characteristics, ensuring proper mechanical design and enclosure protection, incorporating smart battery features, and meeting safety and regulatory requirements.
Q2. How do manufacturers ensure the safety of medical battery packs? Manufacturers implement protection circuits to prevent overcharging and overdischarging, use robust enclosures with appropriate ingress protection ratings, incorporate temperature sensors and cell balancing, and comply with safety standards like UL 1642, IEC 62133, and UN 38.3.
Q3. What is the importance of smart battery features in medical devices? Smart battery features like accurate state-of-charge monitoring, cell balancing, and end-of-life alerts are crucial for ensuring reliable performance, extending battery life, and providing timely information to healthcare providers and patients about the device’s power status.
Q4. How do charge and discharge characteristics affect medical battery performance? Proper charging methods like Constant Current-Constant Voltage (CCCV) and accurate discharge rate calculations are essential for optimizing battery life and ensuring consistent device performance. These characteristics directly impact the reliability and runtime of medical equipment.
Q5. What are the regulatory considerations for medical battery pack design? Medical battery packs must comply with various standards, including FDA-recognized UL and IEC standards for safety. Additionally, manufacturers need to follow proper labeling, traceability, and disposal guidelines to meet regulatory requirements and ensure safe handling throughout the battery’s lifecycle.




