
Navigating the regulatory maze is hard when you work with lithium battery packs and medical devices. You must follow FDA and UN 38.3 standards. If you do not, your product might get recalled. Not following the rules can also stop you from selling your product in some places.
Consequence | Description |
|---|---|
Increased Risk of Recalls | Not following FDA and UN 38.3 standards makes recalls more likely. |
Restricted Market Access | Not meeting these standards can stop your product from being sold or staying in the market. |
You need to follow important standards like IEC 62133, IEC 60086-4, and UL 1642. These help keep your product safe and approved. Staying up to date and acting early helps protect your business. It also helps you keep selling in markets around the world.
Key Takeaways
Follow FDA and UN 38.3 rules to stop product recalls and keep your product in stores.
Do a gap analysis early. This helps you find problems with compliance. It also saves time and money.
Finish all eight UN 38.3 tests. This makes sure lithium batteries ship safely.
Keep your paperwork neat and organized. This helps you answer questions from regulators fast.
Use technology and work with your team. This makes compliance easier and helps you know about new rules.
Part 1: Regulatory Maze Overview
Working with lithium battery packs for medical devices can be confusing. There are many rules and agencies to think about. You need to know how these rules work together. Every step is important for safety and selling your product.
1.1 FDA Compliance Basics
You must follow strict FDA rules before selling lithium battery packs for medical devices. The FDA checks if your batteries are safe and work well. They also look at how you track your products. If you miss something, you might get a Form 483 or Warning Letter. These tell you what problems were found. You have to fix these problems with a CAPA plan. The FDA watches higher-risk devices more closely.
Requirement | Description |
|---|---|
FDA General Safety and Performance Requirements | Batteries must meet the safety rules of IEC 62133, UL 2054, ISO 13485, and IEC 60601-1. |
Biocompatibility | Batteries must be safe to use in the body. |
Safety Features | Batteries need special safety parts for use near people. |
Authentication | Batteries must be checked to stop fake ones. |
Serialization | Batteries must have numbers so you can track them. |
Transportation | Medical batteries must follow all shipping rules. |
Design Features | Batteries need things like overcharge protection, thermal shutdown, and biocompatibility. |
1.2 UN 38.3 Essentials
UN 38.3 is the main rule for shipping lithium battery packs. You must pass eight tests to show your batteries are safe to ship. These tests include vibration, shock, and heat. The tests help stop fires and accidents when shipping. You also need to label, document, and pack batteries by the rules.
The eight tests are:
Altitude simulation
Vibration
Shock
Short circuit
Impact
Overcharge
Forced discharge
Thermal test
Note: Passing UN 38.3 tests shows you care about safety and quality. Most problems happen because of contact loss in the current interruptive device area.
1.3 Scope for Lithium Batteries
The rules cover both primary and secondary lithium batteries. For primary batteries, you must follow IEC 60086-4. For secondary batteries, you must meet IEC 62133. Medical batteries must be made in UL-certified places with good records. UN 38.3 covers batteries shipped alone, in devices, or with devices. You also need to follow new rules for fire labels and shipping on passenger planes.
UN 3090 and UN 3480: For batteries shipped alone.
UN 3091 and UN 3481: For batteries shipped in or with devices.
You must design batteries to stop breaks and short circuits. A strong quality system helps you follow the rules at every step.
Part 2: Compliance Steps
Getting through the rules is easier if you follow clear steps. You need to meet both FDA and UN 38.3 rules. This helps your lithium battery packs for medical devices stay safe and ready for shipping. Each step helps you follow the rules and sell your product.
2.1 Initial Assessment
First, you do a gap analysis. This means you look for missing parts in your plan. You check your papers, tests, and quality systems. You compare them to what the FDA and UN 38.3 want. Industry frameworks can help you with this check.
Framework/Regulation | Description |
|---|---|
Medical Device Regulation (MDR) | Sets safety and clinical evidence standards for medical devices in the EU. |
Food and Drug Administration (FDA) | Categorizes medical devices by risk and sets US safety standards. |
International Standard ISO 13485 | Ensures quality management throughout the device lifecycle. |
Health Insurance Portability and Accountability Act (HIPAA) | Protects patient data in devices that store or transmit information. |
You also look at important safety documents for your products:
Essential Safety Documents | Description |
|---|---|
Cosmetic Product Safety Reports (CPSRs) | Required for EU cosmetics, showing product safety. |
Material Safety Data Sheets (MSDS) | Details hazards and safe handling for electronics and tools. |
FDA and EFSA Approvals | Needed for consumables, confirming compliance with US and EU standards. |
Battery Safety Certifications (UN 38.3) | Confirms lithium battery safety for transport and use. |
Tip: Doing a gap analysis early can save you time and money.
2.2 Documentation
You need to make sure you have all the right papers. For lithium battery packs in medical devices, keep records of tests, certifications, and safety features. Keep your files neat so you can answer questions from regulators fast.
Test Number | Description |
|---|---|
T1 | Altitude simulation |
T2 | Thermal testing |
T3 | Vibration |
T4 | Shock |
T5 | External short circuit |
T6 | Impact and crush |
T7 | Overcharge effects |
T8 | Forced discharge |
Add design details, how to use the product, and tracking records. Make sure your papers match the newest FDA and UN 38.3 rules.
Note: Good paperwork helps you get approved and pass audits.
2.3 Testing for UN 38.3
You must finish all eight UN 38.3 tests for your batteries. These tests show your batteries are safe for shipping and handling. Testing takes about one month. The time depends on battery size and how fast the lab works.
Test Code | Test Name | Description |
|---|---|---|
T1 | Altitude Simulation | Tests battery performance under low-pressure conditions. |
T2 | Thermal Test | Checks stability during extreme temperature changes. |
T3 | Vibration | Assesses resilience to transport vibrations. |
T4 | Shock | Tests ability to withstand impact and acceleration. |
T5 | External Short Circuit | Evaluates safety during short circuit events. |
T6 | Impact/Crush | Assesses tolerance to mechanical stress. |
T7 | Overcharge | Examines protection against overcharging. |
T8 | Forced Discharge | Tests safety under forced discharge conditions. |
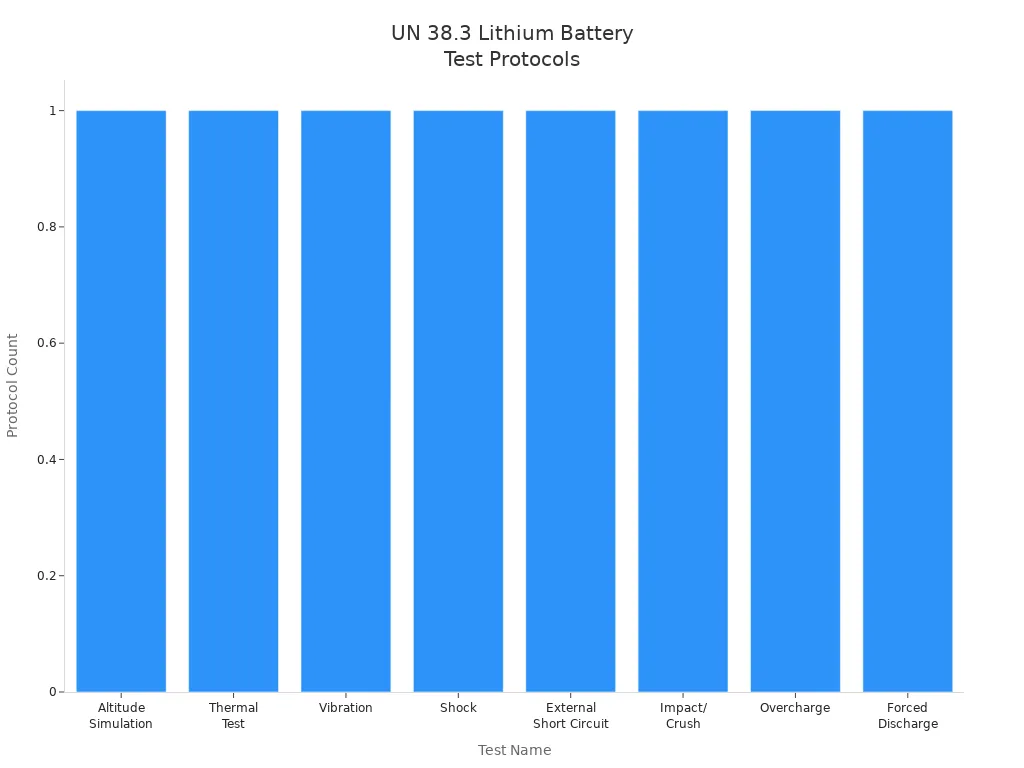
Duration of Testing | Influencing Factors |
|---|---|
About one month | Specific tests required, battery capacity, testing facility efficiency |
Alert: You must test every lithium battery chemistry to meet UN 38.3.
2.4 FDA Submission
You send your papers and test results to the FDA. Your papers must show you follow ANSI/AAMI ES 60601-1 and IEC rules like 62133, 60086 Part 4, and UL 1642. You add design features that stop unapproved batteries and chargers. You give clear steps for storage, charging, and care. You pack batteries to lower fire risk during shipping. You set warehouse rules to stop fires.
Follow ANSI/AAMI ES 60601-1 for safety and performance
Meet IEC 62133, IEC 60086 Part 4, and UL 1642 for lithium batteries
Add design features to block unapproved batteries and chargers
Give strong instructions for storage, charging, and care
Use packaging that lowers fire risk when shipping
Make warehouse rules for safe battery storage
Tip: A full FDA submission helps you get approved faster and sell in more places.
2.5 Monitoring
After approval, you must watch your products and how you work. Use a CAPA system to track problems and fixes. CAPA helps you find what went wrong and stop it from happening again. It helps you keep your products good and your business safe.
CAPA finds problems and stops them from coming back.
It helps you keep making your products better.
CAPA makes sure you follow the rules and protect your business.
Using CAPA in your QMS helps you work better and follow the rules.
Note: Answer FDA questions fast. CAPA is key for long-term quality and following the rules.
Part 3: Challenges
3.1 Navigating the Regulatory Maze
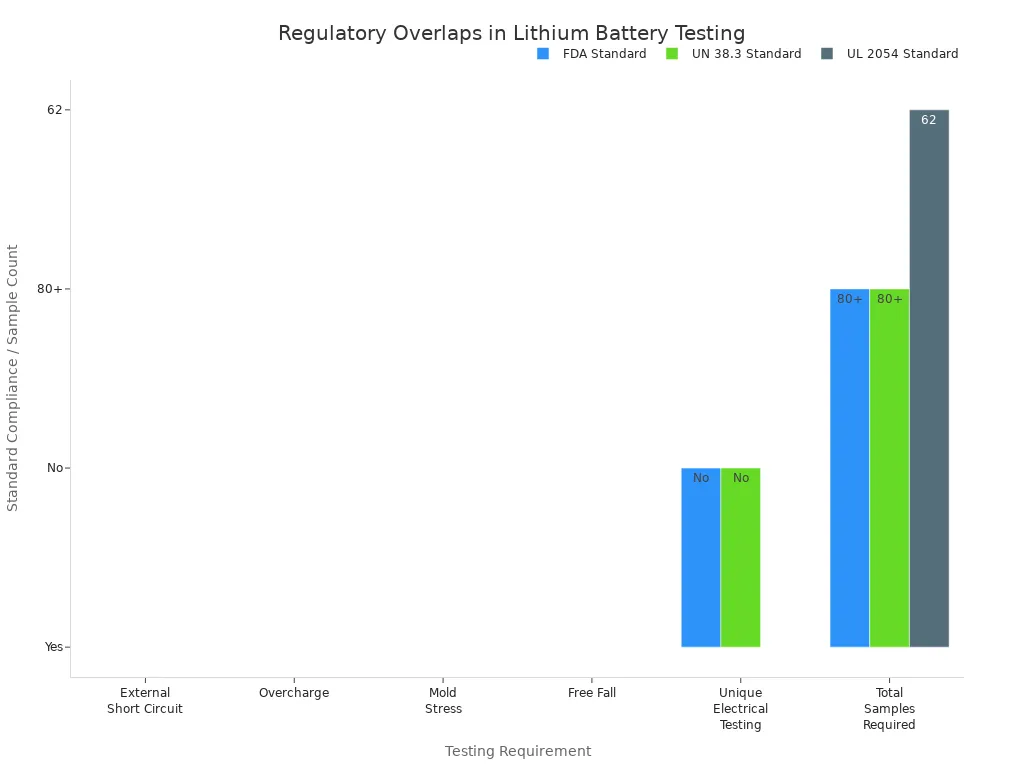
When you work with lithium battery packs for medical devices, rules can be confusing. The FDA, UN 38.3, and UL 2054 all have their own standards. Some tests, like external short circuit, overcharge, and mold stress, are in every standard. But each standard also asks for different things and sample amounts. This makes it harder to follow the rules and can cost more money.
Testing Requirement | FDA Standard | UN 38.3 Standard | UL 2054 Standard |
|---|---|---|---|
External Short Circuit | Yes | Yes | Yes |
Overcharge | Yes | Yes | Yes |
Mold Stress | Yes | Yes | Yes |
Free Fall | Yes | Yes | Yes |
Unique Electrical Testing | No | No | Yes |
Total Samples Required | 80+ | 80+ | 62 |
Tip: You can make things easier by matching your tests and getting help from experts.
3.2 Documentation Pitfalls
Mistakes in paperwork often cause problems with compliance. If records are missing or wrong, you might wait longer for approval or get audited. You can stop these problems by having a strong plan for paperwork:
Teach your team about what documents are needed.
Make clear rules for each job.
Show real examples to help people learn.
Check your records often and give advice.
Train your team to use your document systems.
Note: Training and checking your work often helps you avoid big mistakes.
3.3 Testing Delays
Testing for UN 38.3 can take more time than you think. The rules are hard, there are not many labs, and paperwork can be confusing. Since 2022, you also need to give more details in your test reports, which takes extra time.
Factor | Description |
|---|---|
Complexity of testing | Many steps and strict rules increase processing time. |
Need for certified labs | Fewer options for approved testing slow down scheduling. |
Documentation confusion | Unclear paperwork requirements cause delays. |
You can save time by using better testing tools and working with approved labs. Talking to rule-makers early also helps.
3.4 Supply Chain Issues
Problems in the supply chain can make it hard to follow FDA and UN 38.3 rules. Tougher shipping rules make things cost more and slow down deliveries. If you cannot import or export, you might not get the parts you need for your LiFePO4 Lithium battery or NMC Lithium battery packs.
Evidence Description | Impact on Compliance |
|---|---|
Threatens patient access to essential medical devices | |
Longer delivery times from compliance requirements | Complicates shipping and supply chain management |
Barriers to import and export | Disrupts shipping and access to life-saving devices |
To lower these risks, you should know who your suppliers are, check them often, and use technology to watch for problems. Make risk checks part of starting with new suppliers and review them regularly.
3.5 Cost Risks
If you do not watch your spending, costs can go up fast. Testing, paperwork, and supply chain problems all add to the price. You can keep costs down by using special software, keeping your data in one place, and teaching your team.
Strategy | Description |
|---|---|
Leveraging technology | Use compliance software to automate tasks and improve efficiency. |
Streamlining compliance | Centralize data, audit regularly, and train employees to boost compliance efficiency. |
Integrating risk management | Align compliance with business goals to reduce risks and support growth. |
Technology helps you save money and follow the rules without skipping steps.
Part 4: Best Practices
4.1 Team Collaboration
Good teamwork is important for FDA and UN 38.3 compliance. Make teams with people from engineering, regulatory, and quality jobs. Each person has different skills to help the group. Working together helps you fix problems quickly and make fewer mistakes. In medical and industrial work, teamwork helps with hard lithium battery pack rules. Meet often and talk about updates. Clear talking keeps everyone working together.
Tip: Give people jobs for paperwork, testing, and rule changes. This helps your team stay neat and work well.
4.2 Tech Tools
Technology can help you follow the rules more easily. You can use Product Lifecycle Management (PLM) software to watch product steps and rule needs. PLM helps you see what is happening and make better choices. AI and automation tools help you check battery health and know when to fix things. These tools help stop problems and make batteries work better for LiFePO4 Lithium battery, NMC Lithium battery, LCO Lithium battery, and LMO Lithium battery packs.
Technology Tool | Impact on Compliance Processes |
|---|---|
PLM Software | Makes product steps easier, helps follow rules, lets teams work together, and helps you see what is going on. |
AI and Automation | Helps you use energy better, fix things before they break, watch battery health, and keep medical devices working well. |
Note: Automated tools help you follow tough rules and act fast when rules change.
4.3 Regulatory Updates
You need to know about new rules and standards. Sign up for news from the FDA and other groups. Pick someone to watch for new lithium battery rules. Acting fast helps you not miss any rules. In robotics and security, new rules can change how you make and ship products.
Set alerts for rule changes.
Talk about updates in team meetings.
Change your steps as soon as new rules come out.
4.4 SOPs
Standard Operating Procedures (SOPs) help everyone do things the same way. Write easy SOPs for testing, paperwork, and shipping. Change them when rules change. SOPs help your team follow the right steps every time. You make fewer mistakes and are ready for checks.
4.5 Expert Support
Sometimes you need help from outside your company. Work with consultants who know FDA and UN 38.3 rules well. Experts can look at your work and give good ideas. They help you fix hard problems and get ready for checks. In fast-changing jobs, expert help keeps you ready.
For more on sustainable best practices, visit Our Approach to Sustainability.
Part 5: Resources
5.1 Checklists
You need good checklists to help with FDA and UN 38.3 compliance for lithium battery packs. These lists help you keep track of every step, from the first check to shipping. You can use them for LiFePO4 Lithium battery, NMC Lithium battery, LCO Lithium battery, and LMO Lithium battery packs. Checklists should include paperwork, testing, labels, and shipping. You can get sample checklists from Nature or from industry groups.
Checklist Item | Description | Application Sector |
|---|---|---|
Documentation Review | Make sure all test reports and certificates are ready | Medical, Robotics |
UN 38.3 Test Completion | Check that all eight tests are done | Security, Industrial |
Labeling and Packaging | Use the right fire labels and packaging | Infrastructure, Electronics |
Supplier Audit | Look at supplier records and compliance | All sectors |
Tip: Change your checklists when rules change.
5.2 Online Platforms
You can use online platforms to help with compliance and watch for rule changes. Tools like UL Product IQ and FDA’s CDRH Databases let you look up standards, certificates, and guides. These tools help you check lithium battery pack compliance for medical devices, robots, and security systems.
UL Product IQ: Find certified parts and standards.
FDA CDRH Databases: See device approvals and safety news.
Sustainability Resource: Learn about green battery practices.
Note: Pick platforms that work with your battery type and job area.
5.3 Consultants
You should get help from consultants who know FDA and UN 38.3 rules for lithium battery packs. Experts can help you fix hard compliance problems and get ready for checks. Look for consultants who have worked in medical, industrial, and infrastructure jobs. You can find good contacts in industry groups or through work networks.
Consultant Type | Area of Expertise | Sector Focus |
|---|---|---|
Regulatory Consultant | FDA, UN 38.3, IEC, UL | Medical, Robotics |
Supply Chain Advisor | Import/export, logistics | Industrial, Security |
Sustainability Expert | Conflict minerals, ESG | Infrastructure |
Alert: Ask for proof and read case studies before you hire.
5.4 Training
You need training all the time to keep your team up to date on compliance rules. Training teaches FDA submissions, UN 38.3 testing, and how to do paperwork right. You can pick online classes, webinars, or workshops in person. Training helps your team handle LiFePO4 Lithium battery, NMC Lithium battery, LCO Lithium battery, and LMO Lithium battery pack compliance.
Online courses: FDA, UN 38.3, IEC rules
Webinars: Rule updates and best ways to work
Workshops: Practice testing and paperwork
Training often helps your team make fewer mistakes and follow the rules better.
You can follow FDA and UN 38.3 rules by taking simple steps. Make teams with people from different jobs. Use technology to help you keep things organized. Keep your paperwork neat and easy to find. Watch for new rule changes and train your team often. For LiFePO4 Lithium battery, NMC Lithium battery, LCO Lithium battery, and LMO Lithium battery packs, always check your work and look for ways to get better. This helps you stay ready for changes in medical, robotics, and industrial jobs.
Tip: Keep learning about new rules and check your process often. This keeps your business safe and lets you keep selling your products.
FAQ
What is the difference between LiFePO4 Lithium battery and NMC Lithium battery for medical devices?
Chemistry | Platform Voltage | Energy Density (Wh/kg) | Cycle Life (cycles) |
|---|---|---|---|
LiFePO4 Lithium battery | 3.2 V | 90–160 | 2000+ |
NMC Lithium battery | 3.7 V | 150–220 | 1000–2000 |
LiFePO4 Lithium battery lasts through more charge cycles. NMC Lithium battery stores more energy in the same space.
Do you need to test every battery pack for UN 38.3 compliance?
You have to test each new lithium battery design or chemistry. This means testing LiFePO4 Lithium battery or NMC Lithium battery when you make a new type. You do not need to test every single battery pack. Instead, you test a few samples from each model.
How often should you update compliance documentation?
You should check and update your compliance papers at least once a year. If you change the design, rules, or get new test results, update right away. This keeps your lithium battery packs safe and up to date.
What sectors require the strictest lithium battery compliance?
Medical, robotics, and security systems need the toughest rules. These jobs need strong testing, good paperwork, and tracking for lithium battery packs. This helps keep people safe and makes sure the batteries work well.
Where can you find authoritative resources for lithium battery compliance?
You can get good information from IEC and the FDA. These groups share the latest rules and tips for lithium battery pack compliance in business-to-business jobs.




