 Medical equipment batteries demonstrate significant performance variations, with operational lifespans ranging from several weeks to 15 years depending on chemistry and application requirements. Standard alkaline batteries require replacement every few weeks under regular usage conditions, while lithium-ion configurations typically provide five-year service life. Quality LFP (Lithium Iron Phosphate) batteries extend this performance envelope considerably, delivering operational lifespans exceeding 15 years.
Medical equipment batteries demonstrate significant performance variations, with operational lifespans ranging from several weeks to 15 years depending on chemistry and application requirements. Standard alkaline batteries require replacement every few weeks under regular usage conditions, while lithium-ion configurations typically provide five-year service life. Quality LFP (Lithium Iron Phosphate) batteries extend this performance envelope considerably, delivering operational lifespans exceeding 15 years.
Battery selection for medical devices requires evaluation of multiple technical parameters beyond basic lifespan considerations. Primary cell batteries maintain extended shelf life through low self-discharge characteristics, while secondary batteries generate 90% less waste following twenty recharge cycles. Medical device battery applications demand strict adherence to safety standards and regulatory compliance protocols. ANSI/AAMI ES 60601-1 establishes fundamental safety and performance requirements for medical electrical equipment operating from mains power or battery sources.
Power source selection becomes critical in medical equipment applications where device failure carries significant consequences. Lithium batteries designed for medical devices deliver high energy density, extended operational life, and environmental protection characteristics essential for health monitoring applications. Alternative battery chemistries, including lead-acid and nickel-metal hydride configurations, provide approximately 40% of the charge capacity available from equivalent lithium-based solutions.
The selection process requires systematic evaluation of battery chemistry options, performance specifications, and safety requirements. Critical factors include cycle life characteristics, operating temperature ranges, regulatory compliance standards, and device-specific power requirements. Understanding these technical considerations prevents costly equipment failures, premature battery replacement, and potential safety hazards in healthcare environments where reliable operation remains essential for patient care.
Battery Configuration Requirements for Medical Equipment Applications
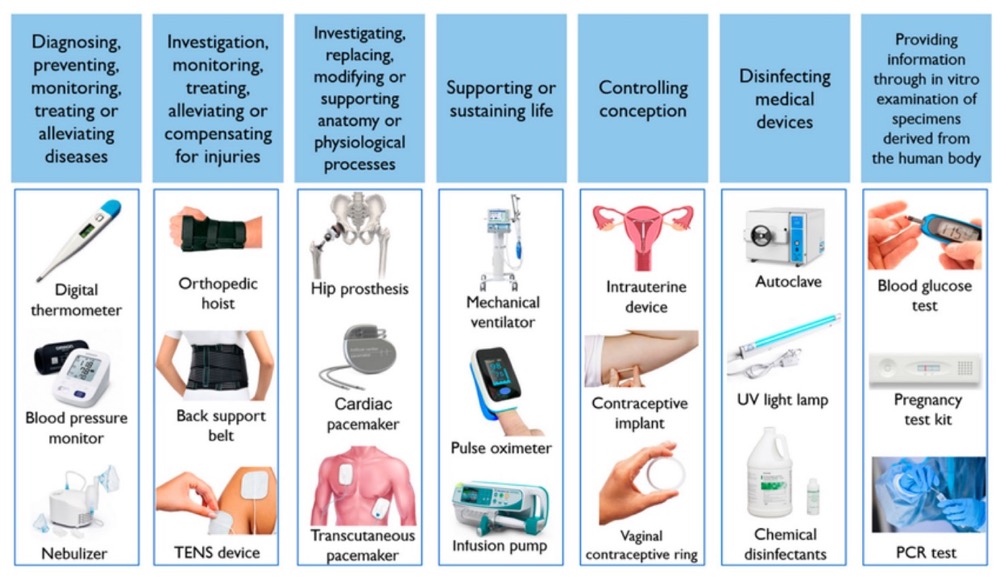 Image Source: ResearchGate
Image Source: ResearchGate
Battery configuration selection determines device performance, maintenance requirements, and operational reliability across medical equipment applications. Each configuration addresses specific power delivery needs, mobility requirements, and service protocols established by healthcare facilities.
Integrated vs. Modular Battery Architecture
Integrated battery systems require complete device disassembly for service access, connecting permanently to stationary medical equipment through internal charging circuits. These configurations optimize space utilization for continuous-operation devices including anesthesia machines, operating room lighting systems, and patient monitoring equipment. The integrated approach eliminates external connection points that could introduce electrical noise or mechanical failure points.
Modular battery systems utilize dedicated access compartments that enable rapid replacement without service disruption. Healthcare personnel can extract depleted batteries and initiate charging cycles while maintaining equipment operation. Portable ultrasound systems, infusion pumps, and handheld diagnostic instruments typically employ modular configurations to support field replacement requirements. This architecture supports continuous operation through battery rotation protocols.
Configuration selection requires evaluation of service intervals, mobility requirements, and acceptable downtime parameters. Modular systems provide operational flexibility at the cost of additional storage and charging infrastructure requirements.
Mobile Cart Power Systems
Medical cart applications require specialized power systems designed for continuous mobility throughout healthcare facilities. Modern LiFeKinnex™ Power Systems eliminate tethering constraints, allowing unrestricted workstation movement without facility power connections.
Lithium Iron Phosphate (LiFePO4) batteries have established dominance in mobile medical cart applications through superior thermal stability characteristics and thermal runaway resistance. These batteries maintain consistent voltage output throughout discharge cycles, preventing equipment damage from power fluctuations. The chemistry provides inherent safety advantages essential for medical environment applications.
Hot-swappable power systems represent advanced cart technology through dual battery configurations that enable seamless power transition during battery exchange. Personnel can replace depleted units without interrupting critical applications or patient care procedures.
Current LiFePO4 technology supports thousands of charge-discharge cycles, delivering significantly extended operational life compared to lead-acid alternatives. This performance reduces replacement frequency and total ownership costs. Manufacturers provide customizable connection interfaces to expand compatibility across diverse medical device requirements.
Emergency Backup Power Solutions
Standby power systems provide critical backup during utility outages, maintaining continuous operation of life-support equipment. These systems maintain full charge status during normal operation, activating immediately upon primary power failure.
Critical care environments including operating theaters and intensive care units require backup power solutions to prevent life-threatening equipment interruptions. Quality standby systems support ventilators, cardiac monitors, dialysis equipment, and additional essential devices during emergency conditions. These systems protect temperature-sensitive biomedical materials including vaccines, blood products, and laboratory specimens from environmental exposure.
Emergency power architectures typically utilize either Uninterruptible Power Supply (UPS) systems or expanded standby power configurations. UPS systems deliver immediate power during outages, eliminating disruption to continuously-operating devices such as oxygen concentrators and ventilators. Larger standby systems incorporate expanded battery banks and generator integration for extended runtime during prolonged outages.
Standby system evaluation requires analysis of runtime specifications, switchover response time, and critical equipment compatibility. Medical-grade UPS systems must comply with UL 60601-1 standards for patient care area applications. Advanced configurations include full isolation transformers, surge protection, and line noise filtering to ensure consistent power delivery to sensitive medical equipment.
Battery Chemistry Selection for Medical Device Applications
“Lithium-ion options offer the highest power availability in the smallest footprint to enable more powerful medical devices and extended battery life in smaller footprints.” — Large Battery, Custom battery manufacturer for medical equipment
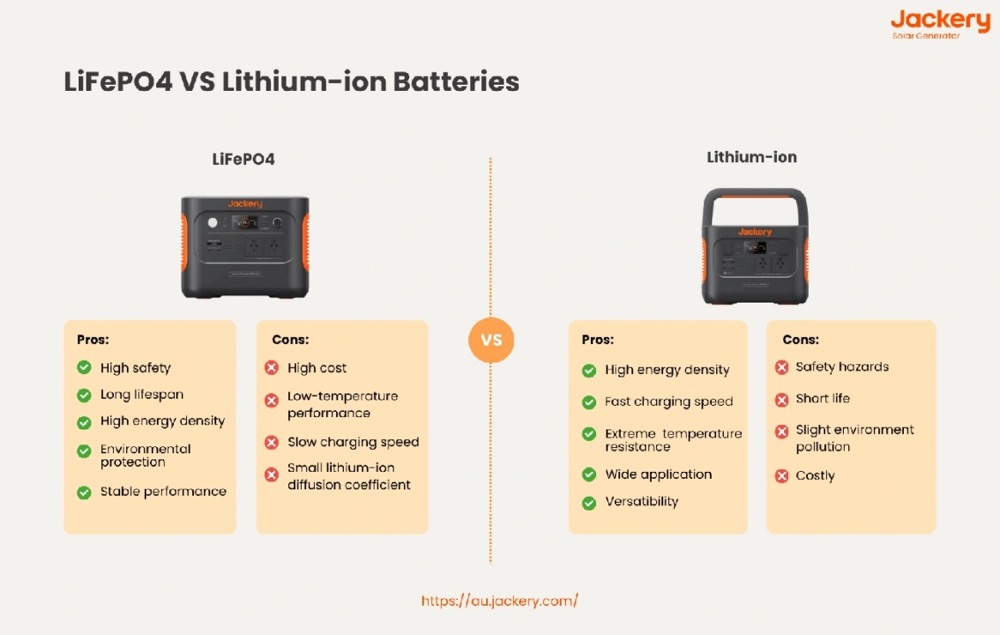 Image Source: Jackery
Image Source: Jackery
Battery chemistry selection determines the fundamental performance characteristics of medical device power systems. Each chemistry presents distinct advantages and limitations that must be evaluated against specific application requirements, safety protocols, and operational environments.
Lithium-Ion and Lithium Iron Phosphate Performance Comparison
Lithium-ion batteries represent the dominant technology in medical device applications, capturing over 60% of the global market as of 2022. The primary driver for this adoption lies in energy density capabilities reaching 250 Wh/kg, which enables significant size and weight reductions in portable medical equipment. However, crimped seal designs present potential failure points, particularly when exposed to sterilization procedures.
LiFePO4 batteries address the thermal stability limitations inherent in standard lithium-ion chemistry. The phosphate-based cathode structure provides superior thermal runaway resistance, a critical safety consideration in medical environments. Additionally, LiFePO4 cells maintain consistent voltage output throughout the discharge cycle, eliminating the voltage droop that can affect sensitive medical electronics. For applications requiring maximum operational reliability, LiFePO4 batteries deliver up to 20 years and 5,000 recharge cycles, making them the preferred choice for critical life-support equipment.
The selection between these lithium chemistries depends primarily on application priorities: lithium-ion for maximum energy density in portable devices, LiFePO4 for maximum safety and longevity in critical stationary equipment.
Nickel-Based Chemistry Trade-offs
NiMH technology offers approximately 95 Wh/kg energy density compared to 39 Wh/kg for NiCd batteries, providing two to three times the capacity in equivalent form factors. This capacity advantage makes NiMH suitable for medical devices requiring extended operation between maintenance cycles.
NiCd batteries compensate for lower energy density through superior environmental tolerance and cycle life performance. These batteries withstand up to 3,000 charge cycles versus approximately 2,000 cycles for NiMH. However, cadmium content creates disposal complications due to toxic heavy metal regulations. The result is a clear preference for NiMH in medical instruments, communication devices, and cost-sensitive applications.
Specialized Chemistries for Compact Medical Devices
Zinc-air batteries achieve exceptional 400 Wh/kg specific energy through ambient air utilization as the cathode material. The flat discharge characteristics and lightweight construction make them suitable for hearing aids, though performance sensitivity to humidity and temperature limits broader applications.
Silver oxide batteries provide stable 1.55V output with excellent discharge characteristics throughout their operational life. The silver content increases cost significantly, but recent developments in silver-coated surfaces show potential infection reduction benefits for implantable medical devices.
Alkaline Batteries in Low-Demand Applications
Alkaline batteries continue to serve effectively in low-power medical devices including glucose meters and digital thermometers. The fundamental limitation lies in high internal resistance that increases as discharge progresses, creating voltage instability under variable loads.
Performance testing demonstrates that small alkaline batteries can experience 8-fold energy delivery differences between minimum (47 Ohm) and maximum (680 Ohm) load conditions. Despite these limitations, alkaline batteries offer acceptable shelf life and operational safety without the regulatory complexity associated with lithium-based chemistries.
Performance Metrics for Medical Battery Selection
 Image Source: MDPI
Image Source: MDPI
Performance evaluation requires systematic analysis of measurable battery characteristics that directly impact medical device functionality. Technical specifications determine application suitability and prevent premature equipment failures in critical healthcare environments.
Cycle Life and Shelf Life Specifications
Cycle life represents the number of complete charge-discharge cycles a battery sustains before capacity degradation exceeds acceptable limits. Performance varies significantly across battery chemistries. Quality LiFePO4 batteries achieve up to 5,000 full recharge cycles, while consumer-grade Li-ion cells typically deliver 500 cycles. Industrial-grade Li-ion batteries provide 20-year operational life with 5,000 complete charge cycles.
Shelf life defines the storage period during which batteries maintain specified performance characteristics. Medical devices supporting life-critical functions require failure rates approaching zero within labeled shelf life parameters. Bobbin-type LiSOCl2 cells maintain 70% of original capacity after 40 years, establishing them as suitable for long-term medical applications.
Self-Discharge Characteristics During Storage
Self-discharge rate quantifies energy loss during inactive periods, often exceeding current requirements for device operation. Battery chemistries demonstrate significant variation in self-discharge performance: bobbin-type LiSOCl2 cells exhibit rates as low as 0.7% annually, industrial Li-ion batteries under 2% yearly, LiFePO4 generally 1-3% monthly, compared to 4-8% monthly for lead-acid configurations.
Temperature conditions substantially influence self-discharge rates through accelerated internal chemical reactions. Medical applications requiring extended storage periods benefit from battery chemistries exhibiting minimal self-discharge characteristics to ensure operational readiness during emergency conditions.
Operating Temperature Specifications and Load Characteristics
Custom lithium ion batteries designed for medical equipment must function across specified environmental conditions. Standard lithium batteries operate within -20°C to 60°C ranges, while specialized low-temperature variants function down to -40°C. Modified LiSOCl2 bobbin cells operate across extreme temperature ranges from -80°C for medical cold chain applications to 125°C supporting autoclave sterilization requirements.
Load profiles define current draw patterns that directly influence battery performance characteristics. High-drain medical devices including infusion pumps and surgical instruments require large current delivery in brief intervals, while monitoring equipment demands consistent, steady output.
Battery Aging and Charging System Considerations
Battery aging and charging protocols significantly impact performance degradation patterns. Deep discharge cycles reduce operational lifespan more than partial discharge cycles—maintaining Li-ion batteries within 20-80% charge ranges can extend cycle life by 30%. Fast charging accelerates degradation through lithium plating mechanisms, with studies indicating batteries charged exclusively with fast chargers retain 70% capacity after 50,000 miles versus 75% for slow-charged alternatives.
Advanced battery management systems (BMS) extend operational lifetimes from 10 to 20 years, reducing total ownership costs by more than 30%. These systems prevent overcharging and over-discharging conditions that can prove catastrophic for medical equipment battery performance.
Regulatory Compliance and Safety Requirements
“Engineers and manufacturers must adhere to specific precautions and safety practices when designing a medical battery.” — Custom Power, Leading custom battery pack manufacturer for medical devices
 Image Source: Batteries Inc.
Image Source: Batteries Inc.
Regulatory compliance represents a fundamental requirement for medical battery implementation, directly affecting both patient safety outcomes and commercial viability. Medical device batteries must meet specific safety standards to ensure reliable operation under clinical conditions while satisfying regulatory requirements for market authorization.
Lithium Battery Safety Standards: IEC 62133 and UL 1642
IEC 62133 establishes comprehensive safety requirements for rechargeable batteries through evaluation of electrical, mechanical, and thermal performance characteristics. Medical device applications typically require compliance with this standard to demonstrate safety verification through standardized testing protocols.
UL 1642, updated to its Sixth Edition in September 2020, addresses safety requirements for both primary and secondary lithium batteries designed for technician-replaceable or user-replaceable applications. The FDA recognizes UL 2054 and UL 1642 as consensus standards for medical devices incorporating lithium batteries. Compliance with these standards facilitates FDA premarket review processes by demonstrating adherence to established safety protocols.
Quality Management: FDA and ISO 13485 Requirements
FDA quality system regulations now align more closely with ISO 13485:2016 through recent amendments to 21 CFR 820. This alignment provides equivalent quality management system assurance while establishing clear performance expectations. ISO 13485 specifies requirements throughout the complete medical device lifecycle, encompassing design and development through production and maintenance phases. Compliance becomes essential for global market access and validates commitment to manufacturing safe, effective medical devices.
Transportation Safety: UN 38.3 Testing Protocol
Lithium batteries require classification as Class 9 dangerous goods during transportation. UN 38.3 testing validates that battery designs can withstand transportation conditions without creating safety hazards. The testing protocol encompasses eight specific evaluations:
- Altitude simulation (50,000 ft atmospheric conditions)
- Thermal cycling (-40°C to 72°C temperature range)
- Vibration resistance testing
- Shock impact evaluation
- External short circuit protection
- Impact and crush resistance
- Overcharge protection verification
- Forced discharge evaluation
Protection Systems: Cell Balancing and Overcharge Prevention
Battery Management Systems (BMS) provide integrated electronic protection through continuous monitoring of voltage, current, temperature, and charge state parameters. Cell balancing maintains uniform charge levels across multiple cells, preventing individual cell degradation that reduces overall battery performance. Overcharge protection prevents excessive heat generation that can cause cell damage or create safety hazards.
These protection mechanisms become particularly critical for lithium battery chemistries, which require precise management for safe operation compared to more tolerant lead-acid alternatives. The BMS integrates multiple safety functions to ensure reliable operation while extending battery service life through optimized charging and discharging control.
Critical Design Errors and Prevention Methods
 Image Source: ScienceDirect.com
Image Source: ScienceDirect.com
Battery pack failures in medical equipment typically result from preventable specification and sourcing errors. The most significant challenges we encounter involve manufacturers attempting to reduce costs through non-certified suppliers, inadequate voltage regulation planning, and insufficient consideration of physical constraints during the design phase.
Non-Certified Battery Manufacturer Selection
Healthcare facilities frequently implement reactive battery replacement strategies, sourcing replacement units only after device failure occurs. Non-OEM battery replacements present substantial risks, including the documented case of a patient monitor that suffered thermal damage due to incompatible battery specifications. Qualified manufacturers must demonstrate compliance with ANSI/AAMI ES 60601-1 standards and maintain UL-certified manufacturing facilities with complete product traceability.
Even experienced procurement teams may overlook critical certification requirements when evaluating cost-competitive alternatives. Verification of manufacturing standards prevents device malfunctions that can compromise patient safety and result in expensive equipment replacement.
Voltage Regulation Compatibility Issues
Medical equipment incorporating sensitive integrated circuits requires precise voltage regulation to prevent malfunction. Battery pack output voltage must remain within specified tolerances throughout the discharge cycle, necessitating either LDO or switching converter regulation. Voltage incompatibility can cause critical errors in medication delivery systems, including incorrect dosing from infusion pumps.
The challenge becomes more complex when batteries age, as internal resistance increases and output voltage characteristics change. Proper voltage regulation design accounts for these variations across the battery’s operational lifespan.
Physical Form Factor Design Constraints
Battery cells experience volumetric expansion during charging cycles, with swelling reaching 7% of original dimensions. Insufficient mechanical clearance creates stress concentrations that damage both battery packs and device enclosures. Medical devices with space-constrained designs often require custom battery shapes—including curved, semicircular, or oval configurations—to maximize capacity within available volume.
The form factor selection process must balance energy density requirements with mechanical design constraints while accounting for thermal expansion and manufacturing tolerances.
Smart Battery Communication Requirements
Advanced medical batteries incorporate sophisticated fuel gaging systems that provide real-time status communication to host devices. Battery capacity calculations vary continuously based on temperature, age, and discharge patterns, requiring dynamic calibration algorithms. Partial discharge cycles introduce measurement errors that drift over time, necessitating periodic recalibration to maintain accuracy.
The complexity of smart battery systems requires early integration planning to ensure proper communication protocols and data accuracy throughout the device’s operational lifetime.
Medical equipment battery selection requires systematic evaluation of technical parameters that directly affect device performance, safety, and operational reliability. The decision process involves balancing energy density requirements, safety protocols, and regulatory compliance standards specific to healthcare applications.
Chemistry selection forms the foundation of performance characteristics. LiFePO4 batteries provide superior thermal stability and extended cycle life for critical care equipment, while lithium-ion configurations deliver optimal energy density for portable devices. Each chemistry offers specific advantages that must align with application requirements and operational environments.
Performance specifications including cycle life, self-discharge rates, and operating temperature ranges must match device load profiles and usage patterns. Portable diagnostic equipment demands different power characteristics than stationary monitoring systems. Understanding these requirements prevents premature failures and operational disruptions in clinical settings.
Regulatory compliance remains mandatory for medical device batteries. Standards including IEC 62133, UL 1642, and ISO 13485 establish safety requirements and facilitate market approval processes. Battery management systems with proper cell balancing and overcharge protection provide essential safeguards for lithium-based power sources.
Common specification errors include voltage compatibility mismatches, inadequate expansion allowances, and non-certified manufacturer selection. These oversights result in equipment failures, safety hazards, and increased replacement costs. Proper manufacturer qualification and technical specification verification prevent these issues.
Battery selection decisions affect long-term operational costs, maintenance requirements, and device reliability. Quality power sources extend equipment lifespan, reduce maintenance frequency, and ensure consistent performance in clinical applications where reliability remains essential for patient care outcomes.
Key Takeaways
Selecting the right medical equipment battery is crucial for patient safety and operational efficiency, with proper choices preventing costly failures and ensuring reliable performance when lives depend on it.
- Choose certified manufacturers only– Always verify compliance with ANSI/AAMI ES 60601-1 standards and use UL-certified factories to prevent device malfunctions and safety incidents.
- Match battery chemistry to application needs– LiFePO4 batteries offer superior safety and 5,000+ cycles for critical equipment, while lithium-ion provides highest energy density for portable devices.
- Verify voltage compatibility with device ICs– Incompatible voltage regulation can cause equipment malfunctions and potentially dangerous situations like incorrect medication dosing.
- Account for battery expansion during charging– Batteries can swell up to 7% during charging, requiring proper space allocation to prevent mechanical stress and damage.
- Prioritize regulatory compliance early– Obtain IEC 62133, UL 1642, and ISO 13485 certifications to ensure legal marketability and streamline FDA approval processes.
Quality medical batteries deliver long-term value through extended device lifespan, reduced maintenance costs, and most importantly, reliable performance during critical healthcare procedures. Investing in proper battery selection today prevents expensive equipment failures and safety hazards tomorrow.
FAQs
Q1. What are the key factors to consider when selecting batteries for medical equipment? The main factors include battery chemistry, energy density, voltage compatibility, cycle life, operating temperature range, safety certifications, and regulatory compliance. It’s crucial to match these characteristics to the specific medical device requirements and usage patterns.
Q2. How do lithium-ion and LiFePO4 batteries compare for medical applications? Lithium-ion batteries offer higher energy density, making them ideal for portable devices. LiFePO4 batteries provide superior thermal stability and longevity, with up to 5,000 recharge cycles, making them suitable for critical equipment like ventilators and monitoring systems.
Q3. Why is regulatory compliance important for medical equipment batteries? Regulatory compliance ensures patient safety and legal marketability. Certifications like IEC 62133, UL 1642, and ISO 13485 validate safety standards and streamline FDA approval processes. Compliance is crucial for global market access and demonstrates commitment to manufacturing safe, effective medical devices.
Q4. What are common mistakes to avoid when selecting medical equipment batteries? Common mistakes include using non-certified manufacturers, ignoring voltage compatibility with device ICs, overlooking battery expansion during charging, and underestimating power requirements for smart medical batteries. These errors can lead to device malfunctions, safety hazards, and costly equipment failures.
Q5. How does battery management impact the performance of medical equipment? Advanced battery management systems (BMS) can significantly extend battery lifetimes, potentially from 10 to 20 years, reducing total ownership costs by over 30%. BMS prevents overcharging and over-discharging, ensures proper cell balancing, and provides critical data communication between the battery and the medical device.




