You rely on stable power from lithium batteries to ensure your laboratory scales deliver precise results every time. Measurement accuracy depends on consistent battery output, as even small fluctuations can introduce errors. Recent studies show that accurate real-time estimation of a battery’s state of health helps maintain reliable performance, reducing measurement volatility. By following best practices and using reliable testing methods, you strengthen your ability to achieve repeatable, trustworthy outcomes in your lab.
Key Takeaways
Stable power from lithium batteries is essential for accurate laboratory measurements. Monitor battery voltage to avoid errors.
Use lithium batteries within their recommended temperature range to maintain consistent voltage and improve measurement precision.
Implement regular calibration checks on testing tools to ensure accurate battery performance and reliable data.
Utilize prediction models to analyze battery health and optimize remaining useful life, reducing downtime in laboratory operations.
Choose lithium-ion batteries with high energy density and long cycle life to enhance efficiency and reliability in your lab.
Part 1: Stable Power and Measurement Accuracy

1.1 Power Fluctuations and Errors
You depend on stable power to achieve reliable measurements in your laboratory. When you use lithium batteries, you expect consistent output. If the battery voltage fluctuates, your scale may show different results for the same sample. This drift can lead to costly mistakes and wasted resources. You notice that unstable power causes errors, especially when the battery state of charge drops or the temperature changes.
Tip: Always monitor the battery voltage during critical measurements. Even small changes can affect your results.
The stability of lithium batteries depends on several factors. Temperature and state of charge play a major role. You can see how voltage stability changes under different conditions in the table below:
Temperature (°C) | State of Charge (SoC) | Voltage Stability Observations |
|---|---|---|
25 | 20% | More stable OCV behavior |
45 | 20% | Increased fluctuations observed |
10 | N/A | Smoother OCV behavior |
When you operate lithium batteries at higher temperatures, you observe more voltage fluctuations. These fluctuations reduce the repeatability of your laboratory scale results. Lower temperatures and moderate state of charge help maintain stable power and improve measurement consistency.
1.2 Consistent Voltage for Precision
You need consistent voltage to maintain precision in your laboratory scale measurements. Lithium batteries offer stable power when you use them within their recommended operating range. The resolution of your measurement system sets the minimum voltage consistency required. For example, if your battery cycler has a resolution of 150 µV, you cannot detect voltage changes smaller than this value. If the battery voltage drops below this threshold, your scale may miss critical changes, leading to errors in measurements such as cut-off voltage during charging.
You achieve the highest accuracy when the battery voltage remains steady and matches the resolution of your equipment. Consistent voltage output from lithium batteries ensures that your laboratory scale provides repeatable and trustworthy results. You improve performance by selecting batteries with proven stability and by controlling environmental factors like temperature.
Use lithium batteries with high voltage stability for your laboratory scales.
Keep the battery within the recommended temperature range.
Monitor the state of charge to avoid voltage drops.
Stable power from lithium batteries is the foundation for accurate laboratory measurements. You protect your workflow and data integrity by choosing the right battery and maintaining optimal conditions.
Part 2: Batteries and Technical Advantages

2.1 Lithium Battery Features
You need stable power for your laboratory scales, and lithium-ion battery technology delivers this with advanced engineering. The structure of a lithium-ion battery includes a negative electrode (anode) and a positive electrode (cathode), separated by a porous separator. This design allows lithium ions to move efficiently during charge and discharge cycles. You benefit from a safe operating voltage range, which protects battery capacity and ensures consistent output. The separator prevents direct contact between electrodes, improving safety and stability.
Rapid innovation in lithium-ion battery modeling drives new demands in laboratory environments.
You optimize battery capacity and safety by understanding the physical and electrochemical properties of lithium.
You must address challenges such as thermal runaway to maintain stable power delivery.
You see lithium-ion battery packs used in medical devices, robotics, security systems, infrastructure, consumer electronics, and industrial applications. These industries rely on battery capacity and voltage consistency for reliable performance.
Battery Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) | Application Scenarios |
|---|---|---|---|---|
Lithium Iron Phosphate | 3.2 | 90–160 | 2,000+ | Medical, Industrial, Robotics |
Lithium Nickel Manganese Cobalt (NMC) | 3.7 | 150–220 | 1,000–2,000 | Security, Infrastructure, Consumer Electronics |
Lithium Cobalt Oxide | 3.6 | 150–200 | 500–1,000 | Consumer Electronics |
2.2 Solid-State Battery Innovations
You gain even greater stability with solid-state lithium-ion battery technology. These batteries use a solid polymer electrolyte, which improves safety and voltage consistency. You can expect over 2,000 cycles at 1C/1C using NMC811 cathodes. The operational temperature range stretches from -20° to +60°C, making these batteries suitable for demanding environments. You see solid-state lithium-ion battery packs passing nail penetration and thermal runaway tests, which means you get enhanced safety for your laboratory scales.
Feature | Specification |
|---|---|
Cell Types | 1Ah and 5Ah pouch cells (Fall 2025) |
Electrolyte Type | Solid polymer electrolyte (SPE) |
Cycle Life | Over 2,000 cycles at 1C/1C (NMC811 cathodes) |
Operational Temperature Range | -20° to +60°C |
Safety Compliance | Nail penetration, thermal runaway tests passed |
Manufacturing Location | USA |
Application Areas | Electric vehicles, aerospace, defense |
Future Developments | Larger 10–20Ah cells expected |
You notice that solid-state lithium-ion battery modeling shows improved voltage consistency compared to traditional lithium-ion battery packs. Advances in materials science and engineering strategies enhance battery capacity and overall performance.
Solid-state lithium-ion battery architecture uses solid electrolytes for better voltage consistency.
You see unique battery characteristics that support stable power delivery in laboratory scales.
2.3 Comparison with Other Batteries
You need to compare lithium-ion battery packs with other battery types to understand their advantages. Lithium-ion battery modeling shows a flat discharge curve, which means you get consistent voltage output throughout the cycle. Other chemistries, such as nickel-metal hydride, show more pronounced voltage drops and higher self-discharge rates.
Battery Type | Discharge Curve Characteristics |
|---|---|
Lithium Batteries | Flat discharge curve, consistent voltage output |
Other Chemistries | More pronounced voltage drop during discharge |
Battery Type | Discharge Curve Characteristics |
|---|---|
Lithium Batteries | Relatively flat voltage plateau during initial discharge |
Other Chemistries | Varying discharge profiles with less voltage stability |
Battery Type | Discharge Curve Characteristics |
|---|---|
Lithium Iron Phosphate (LFP) | Flat discharge curve, stable performance throughout discharge |
Other Chemistries | Less consistent voltage output during discharge |
Feature | Lithium-Ion | Nickel-Metal Hydride |
|---|---|---|
Self-discharge | ~2–3%/month | ~15–25%/month |
Specific power | ~200–300+ W/kg | ~100–200 W/kg |
Cycle life to ~80% | ~500–2,000+ cycles | ~300–1,000 cycles |
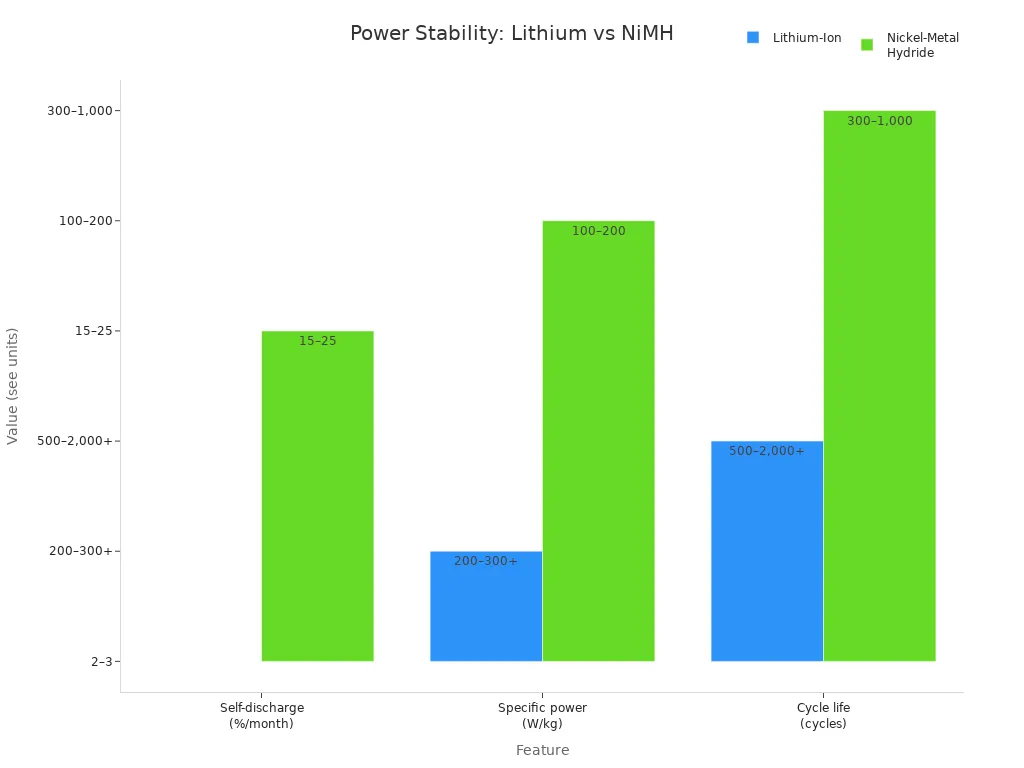
You see that lithium-ion battery packs outperform nickel-metal hydride batteries in laboratory scales. You get lower self-discharge, higher specific power, and longer cycle life. You rely on lithium-ion battery modeling to maximize battery capacity and maintain stable power for accurate laboratory results.
Part 3: Best Practices for Stable Power
3.1 Calibrated Testing Tools
You achieve stable power in your laboratory by using calibrated testing tools for current discharge assessments. Calibration ensures your equipment meets strict performance specifications. Over time, even the best instruments can drift, causing errors in your lithium-ion battery measurements. When you use out-of-calibration tools, voltage errors can affect your capacity readings and disrupt your energy storage data. You should follow recognized standards for calibration in laboratory settings:
Standard | Description |
|---|---|
UN/DOT 38.3 5th Edition, Amendment 1 | Recommendations on the Transport of Dangerous Goods |
IEC 62133-2:2017 | Safety requirements for portable sealed secondary lithium cells, and for batteries made from them, for use in portable applications – Part 2: Lithium systems |
UL 2054 2nd Edition | Household and Commercial Batteries |
Tip: Schedule regular calibration checks to maintain the accuracy of your lithium-ion battery testing and support your energy storage management.
3.2 Cell Preparation and Maintenance
You improve lithium-ion battery stability by focusing on cell preparation and maintenance. Uniformity and dryness in electrode preparation are critical for reliable energy storage and stable power. You should:
Mix slurry with the right equipment and timing for consistent electrode quality.
Pre-grind and sieve solid powders before wet mixing with the binder solution.
Maintain consistent solid content across batches for quality control.
Control moisture content to prevent gas evolution and safety risks.
Dry the separator before use to ensure effective lithium-ion battery operation.
Check moisture levels regularly to protect the structure of active materials.
You support responsible sourcing and sustainability by following best practices. Learn more about sustainability and conflict minerals in lithium battery manufacturing.
3.3 Reliable Electrochemical Methods
You rely on robust electrochemical testing methods to evaluate lithium-ion battery stability. Laboratory setups like beaker cells, Swagelok cells, and coin cells each offer unique advantages. Beaker cells are easy to assemble but use more electrolyte. Swagelok cells maintain pressure well and are simple to construct. You use half-cell configurations with precise counter and reference electrodes to assess new lithium materials.
Versatile electrochemical methods such as cyclic voltammetry, electrochemical impedance spectroscopy, and charge/discharge testing give you detailed insights into battery performance and degradation. Standardizing your testing protocols and developing robust electrochemical modeling approaches help you achieve reproducible results in energy storage research. You can enhance your battery management system by integrating these methods—see more about battery management systems.
Part 4: Practical Benefits for Labs
4.1 Reduced Downtime
You want your laboratory to run smoothly without interruptions. Lithium-ion battery packs help you achieve this by providing stable power for your scales and instruments. In medical labs, robotics testing centers, and security system facilities, you see fewer unexpected shutdowns when you use lithium-ion battery packs. Consistent voltage output means your equipment stays online longer, and you avoid delays in your workflow. You rely on accurate prediction of remaining useful life to schedule maintenance before failures occur. This approach improves prediction accuracy and keeps your lab productive.
Note: You can use prediction models to analyze battery data and forecast remaining useful life. This helps you plan replacements and avoid downtime.
4.2 Longer Battery Life
You benefit from lithium-ion battery packs with extended cycle life. In industrial labs and infrastructure testing, you need batteries that last through many charge and discharge cycles. Lithium-ion battery packs offer higher energy density and longer remaining useful life compared to other chemistries. You use prediction models to monitor battery health and optimize usage. Accurate prediction of remaining useful life allows you to maximize battery performance and reduce costs.
Battery Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) | Application Scenarios |
|---|---|---|---|---|
Lithium Iron Phosphate | 3.2 | 90–160 | 2,000+ | Medical, Industrial, Robotics |
Lithium Nickel Manganese Cobalt (NMC) | 3.7 | 150–220 | 1,000–2,000 | Security, Infrastructure, Consumer Electronics |
Lithium Cobalt Oxide | 3.6 | 150–200 | 500–1,000 | Consumer Electronics |
You use data from prediction models to track battery health and remaining useful life. This helps you make informed decisions about replacements and maintenance.
4.3 Improved Workflow
You improve your workflow by using lithium-ion battery packs with reliable prediction of remaining useful life. In consumer electronics labs and industrial environments, you depend on stable power to produce accurate results. Prediction models analyze battery data and provide early warnings about potential failures. You adjust your workflow based on prediction accuracy and remaining useful life forecasts. This proactive approach reduces errors and improves your lab’s efficiency.
You use lithium-ion battery packs for stable power in medical, robotics, security, infrastructure, consumer electronics, and industrial labs.
You rely on prediction models and battery data to optimize remaining useful life and achieve accurate results.
Tip: You can access authoritative research on lithium-ion battery prediction accuracy and remaining useful life in Nature.
You achieve accurate laboratory scale results when you use lithium battery packs that deliver stable power. Lower RMSE, MAE, and MAPE values show how stability improves measurement accuracy:
Statistic | Description | Impact on Accuracy |
|---|---|---|
RMSE | Overall data dispersion; smaller is better | Higher stability, better accuracy |
MAE | Actual predicted error; smaller is better | More accurate measurements |
MAPE | Relative error in %; smaller is better | Reliable results |
Technical features like high energy density, long life span, and improved safety—especially in solid-state designs—support your lab’s performance. You can further reduce challenges by optimizing cell configuration and testing protocols.
Consider adopting lithium battery packs and best practices to maximize accuracy and efficiency in your laboratory operations.
FAQ
What makes lithium battery packs ideal for laboratory scales?
Lithium battery packs deliver stable voltage and long cycle life. You get consistent measurements and reduced downtime. Their high energy density supports extended use in demanding lab environments.
How do you maintain stable power in lithium battery packs?
You monitor battery voltage and temperature. You use calibrated testing tools and follow best practices for cell preparation. Regular maintenance ensures reliable performance and accurate results.
Which lithium battery chemistry offers the longest cycle life?
Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) |
|---|---|---|---|
Lithium Iron Phosphate (LFP) | 3.2 | 90–160 | 2,000+ |
NMC | 3.7 | 150–220 | 1,000–2,000 |
Lithium Cobalt Oxide (LCO) | 3.6 | 150–200 | 500–1,000 |
You achieve the longest cycle life with Lithium Iron Phosphate (LFP) packs.
How do prediction models improve battery management in labs?
Prediction models analyze battery data. You use these models to estimate remaining useful life. This helps you plan maintenance and replacements, reducing unexpected downtime.
Where can you find authoritative research on lithium battery performance?
You access reliable studies in journals like Nature Energy. These sources provide accurate data on lithium battery packs and their application in laboratory environments.




