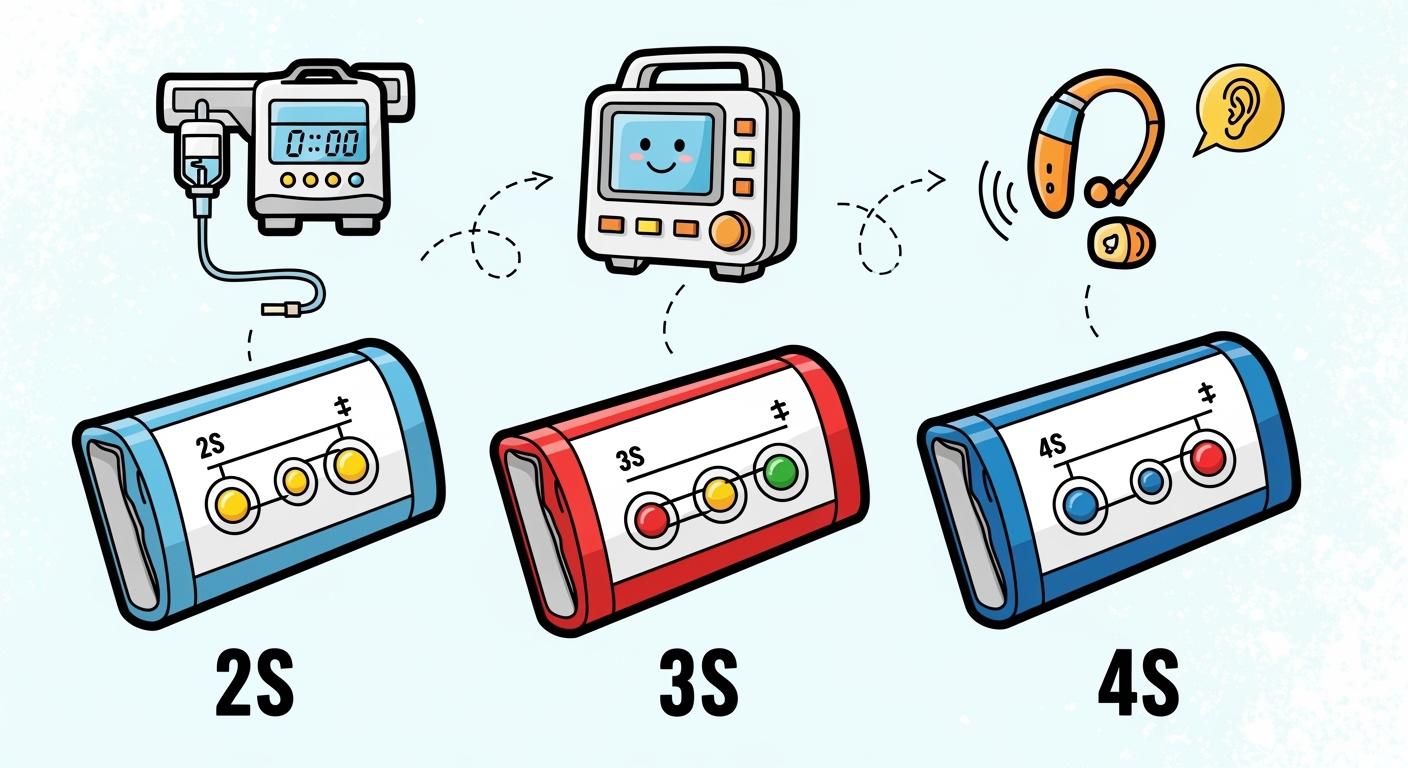
You need to match lithium battery architectures to your device’s voltage and power demands. 2S, 3S, and 4S packs offer increasing nominal voltages, supporting everything from low-power monitors to high-performance implantable medical equipment. Recent trends show a move toward ultra-thin, flexible battery designs and advanced safety features for enhanced reliability.
Lithium-ion batteries now dominate the medical market.
Companies focus on compact, durable, and high-capacity battery solutions.
Key Takeaways
Choose the right battery architecture (2S, 3S, 4S) to match your device’s voltage needs for efficient operation.
Ensure safety by selecting batteries with advanced protection features like cell balancing and temperature monitoring.
Verify that battery packs meet regulatory standards for medical devices to ensure safety and reliability.
Part 1: Lithium Battery Architectures Overview
1.1 2S, 3S, 4S Configurations Explained
You encounter three main lithium battery architectures in professional environments: 2S, 3S, and 4S. Each configuration refers to the number of cells connected in series, which directly affects voltage and power output. The 2S battery uses two cells, the 3S battery uses three, and the 4S battery uses four. This series connection increases the total voltage available for your devices.
Tip: Choosing the right architecture ensures your equipment operates efficiently and safely.
Here is a quick comparison:
Configuration | Voltage (V) |
|---|---|
2S | 7.4 |
3S | 11.1 |
4S | 14.8 |
1.2 Voltage and Power Output
You must match the voltage and power output of lithium battery architectures to your device’s requirements. The 2S battery delivers 7.4V, suitable for low-power medical devices and compact equipment. The 3S battery provides 11.1V, supporting moderate loads and higher performance. The 4S battery reaches 14.8V, ideal for advanced medical equipment and industrial applications. For example, a 3S battery powering a 300-watt motor draws 27A, while a 4S battery draws only 20.3A for the same load, improving efficiency.
Note: The 4S architecture offers a nominal voltage of 14.8V (3.7V per cell), with a maximum charging voltage of 16.8V and robust overcharge protection. You benefit from higher energy density and longer runtime.
1.3 Typical Medical Device Applications
You see lithium battery architectures used across various sectors. In medical applications, 2S batteries power portable monitors and diagnostic devices. 3S batteries support infusion pumps and imaging equipment. 4S batteries drive high-performance medical equipment, such as surgical robots and advanced imaging systems. In robotics, security systems, infrastructure, consumer electronics, and industrial settings, these architectures provide tailored solutions for each application.
You select the architecture based on voltage needs, device size, and operational demands. Lithium batteries offer flexibility, safety, and reliability for your equipment.
Part 2: Technical Differences and Design
2.1 Series Configuration and BMS Complexity
When you design medical devices with 2S, 3S, or 4S lithium battery packs, you must address the complexity of series configurations and the battery management system (BMS). Each additional cell in series increases the voltage and the need for precise control. The table below highlights key technical challenges:
Challenge Type | Description |
|---|---|
Protection Logic Conflicts | Using individual BMS for each pack in series can cause conflicts, leading to power failures. |
Safety Risks | Malfunctioning BMS may not cut off, risking overcharging and hazards such as fire. |
Cost and Wiring Complexity | High-current BMS units cost more and require thicker cables, complicating installation. |
Cell Imbalance | Active balancing in the BMS is essential to prevent cell imbalance and extend battery life. |
Consistency Requirements | All BMS units in parallel must match in brand and model for uniform protection parameters. |
You must select a BMS that matches your application and equipment requirements. Advanced BMS features, such as overcharge protection and cell balancing, are critical for medical performance and safety.
2.2 Size, Weight, and Integration
You need to balance size, weight, and integration when selecting lithium batteries for medical devices. Higher series counts, such as 4S, increase voltage but may add bulk. Compact devices benefit from LiPo chemistries, which offer flexible packaging and lightweight construction. Battery life constraints shape device design, influencing component selection and wireless features. You should consider the form factor and integration strategy early in the design process to ensure optimal performance.
Tip: Choose battery shapes and chemistries that fit your device’s footprint and operational needs.
2.3 Energy Delivery Stability
Stable energy delivery is essential for medical equipment. Lithium batteries provide consistent discharge voltage and long cycle life, supporting high-performance devices. You benefit from a robust battery management system that offers overcharge protection, state of charge estimation, and temperature monitoring. Key factors influencing stability include:
High-current pulse capability for demanding application scenarios.
Safety features such as waterproofing and explosion-proof construction.
Mechanical and electrical design for shock resistance and heat dissipation.
Use of high-strength nylon with fiberglass reinforcement for structural reliability.
A certified smart battery management system ensures compliance and safety, especially in critical medical environments.
Part 3: Power and Performance in Medical Devices

3.1 Voltage Needs for Medical Devices
You must select the correct voltage for medical devices to ensure reliable operation and safety. Most lithium-ion cells deliver a nominal voltage of 3.7V, with a full charge at 4.2V and a discharge cut-off between 3.0V and 2.8V. Maintaining these voltage limits supports battery safety and extends battery life. Stable voltage output prevents diagnostic drift and sensor error in safety-critical equipment. You should always verify voltage requirements for your application before integrating lithium batteries.
Voltage Requirement | Description |
|---|---|
Nominal Voltage | 3.7V |
Full Charge Voltage | 4.2V |
Discharge Cut-off | 3.0V – 2.8V |
3.2 Runtime and Load Performance
You need to balance runtime and load performance when choosing a battery for medical equipment. Lithium polymer batteries maintain a stable voltage across most of their charge cycle, supporting consistent device operation. Longer runtime reduces downtime and increases efficiency in medical and industrial settings. You should consider cycle life, energy density, and load demands for each battery chemistry, such as LiFePO4, NMC, LCO, LMO, and LTO. Proper battery safety protocols, including advanced battery management systems, help you avoid risks and ensure compliance.
Maintains stable voltage output for over 90% of charge.
Prevents sensor error in medical devices.
Supports continuous operation in robotics and security systems.
3.3 High Power vs. Low Power Applications
You must match battery architecture to your application’s power needs. High-power batteries feature low areal cathode capacity and high porosity, while high-energy batteries focus on energy density but may degrade faster at high C-rates. NCM batteries offer higher power density but present safety risks and shorter lifespan. LFP batteries provide longer cycle life and improved safety, though efficiency drops in cold environments.
Battery Type | High Power Characteristics | Low Power Characteristics |
|---|---|---|
Lithium-ion | High C-rate, low areal capacity, high porosity | Designed for energy density, may degrade faster at high C-rates |
NCM | Higher energy density, better cold performance | Shorter lifespan, thermal instability risks |
LFP | Safer, longer cycle life | Efficiency drops in cold temperatures |
Note: You should consider sustainability and responsible sourcing when selecting lithium batteries. Review our sustainability page and conflict minerals statement for more information.
Part 4: Safety and Compatibility
4.1 Safety Features in Battery Packs
You must prioritize safety when selecting lithium batteries for medical equipment. Each battery architecture—2S, 3S, and 4S—integrates advanced safety features to protect both devices and patients. Key safety mechanisms include:
Cell balancing function, which ensures each cell charges evenly and extends battery life.
Real-time cell state monitoring, which checks voltage, current, and temperature, activating protection if thresholds are exceeded.
Auxiliary protection components, such as PTCs for over-temperature protection, fuses for irreversible protection, and NTC sensors for thermal shutdown.
A robust battery management system (BMS) supports these features. The BMS monitors cell voltage, current, and temperature to prevent overcharging, over-discharging, and thermal runaway. It can reduce charging current or terminate charging when voltage limits approach. The BMS also requests connected loads to reduce current demands when voltage nears minimum thresholds. Thermal management remains crucial, as it directly impacts performance, lifespan, and safety.
Parameter | Description |
|---|---|
Cell Voltage | Prevents overcharging or over-discharging, which can damage the battery. |
Current | Ensures charging/discharging stays within safe limits. |
Temperature | Prevents performance loss and thermal risks by monitoring heat levels. |
Tip: Always verify that your battery pack includes these safety features before integrating it into medical devices.
4.2 Device Compatibility and Compliance
You must ensure that lithium battery packs meet strict regulatory and compatibility requirements for medical devices. Regulatory frameworks such as the FDA and European MDR require compliance with essential safety and performance standards. Key requirements include:
Essential safety and performance compliance (MDR Annex I).
Biocompatibility for patient safety.
Adherence to ISO 13485 quality management systems.
Thorough testing and evaluation for all battery packs.
In the U.S., you must follow FDA design controls, risk management, and quality system regulations. For Class II medical devices, you need to document and test battery subsystems under 510(k) or PMA processes. Compliance with standards like UL 2054, UL 1642, IEC 60601, IEC 62133, and UN 38.3 ensures electrical safety and reliable operation.
Safety Feature | Description |
|---|---|
Overcharge protection | Prevents exceeding safe voltage levels. |
Over-discharge protection | Ensures battery does not fall below safe discharge levels. |
Short circuit detection | Identifies and mitigates short circuit conditions. |
Cell balancing | Maintains equal charge across all cells. |
Temperature monitoring | Prevents overheating and ensures safety. |
Adheres to standards for safety and reliability in medical devices. |
You should confirm that your battery architecture supports all required safety features and regulatory standards before deploying it in medical equipment.
You must weigh energy density, cycle life, safety, and regulatory compliance when selecting a lithium battery architecture for medical devices. Consult with battery and device manufacturers to address power, size, and integration needs. For optimal results, implement robust quality assurance and battery management systems during device development or procurement.
FAQ
What makes 2S, 3S, and 4S lithium battery packs suitable for medical device monitoring?
You gain stable voltage and reliable monitoring with multi-cell battery packs. These architectures support real-time monitoring, long cycle life, and robust protection for medical, robotics, and industrial applications.
How does protection architecture differ between lithium battery chemistries in multi-cell battery packs?
You see LiFePO4, NMC, LCO, LMO, and LTO chemistries use advanced protection. Each protection architecture includes cell balancing, temperature monitoring, and overcharge protection for safe operation in medical and security systems.
Can Large Power provide custom protection solutions for medical device monitoring?
You can request custom protection solutions from Large Power for medical device monitoring. Visit Large Power custom battery solution for tailored multi-cell battery packs with advanced protection and monitoring features.
Battery Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Long Cycle Life | Protection Features |
|---|---|---|---|---|
LiFePO4 | 3.2 | 90-160 | Yes | Cell balancing, thermal |
NMC | 3.7 | 150-220 | Yes | Overcharge, monitoring |
LCO | 3.7 | 150-200 | No | Over-discharge, fuse |
LMO | 3.7 | 100-150 | Yes | Temperature, fuse |
LTO | 2.4 | 70-80 | Yes | Real-time monitoring |
Tip: You should always verify protection features and monitoring capabilities before integrating multi-cell battery packs into medical or industrial devices.




