Miniaturization, biocompatibility, and longevity present key challenges for batteries for implantable medical devices. Engineers must design power sources that fit within strict size limits and maintain high performance. Patient safety depends on reliable energy delivery and materials that do not trigger immune responses. The industry now favors advanced lithium-based solutions for implantable devices, replacing outdated chemistries and improving device reliability.
Key Takeaways
Miniaturization is crucial. Engineers must design batteries that fit in small spaces without losing performance. Smaller batteries enhance device usability.
Biocompatibility is essential for patient safety. Choosing materials that do not trigger immune responses ensures the reliability of implantable devices.
Longevity impacts costs and patient care. Batteries that last longer reduce the need for replacements, benefiting both patients and healthcare systems.
Advanced lithium-based chemistries improve performance. These options provide higher energy density and longer life, making them ideal for medical applications.
Collaboration drives innovation. Partnerships among manufacturers, battery experts, and scientists lead to better solutions for implantable devices.
Part1: Batteries for Implantable Medical Devices
1.1 Miniaturization
Miniaturization remains a primary challenge for batteries for implantable medical devices. Engineers must design power sources that fit inside extremely compact spaces without sacrificing energy density or reliability. The smallest commercially available batteries, such as the Contego 1.5 mAh, measure only 0.299 inches in length and 0.114 inches in diameter. These batteries feature hermetically sealed titanium cases and thermal shutdown separators, supporting advanced neuromodulators and monitors. Micro batteries, like those from EaglePicher, offer further reductions in size for neurostimulation applications.
Note: The size of the battery directly impacts the overall design and functionality of the implantable device. Traditional lithium-based chemistries, including lithium metal and lithium-ion (LCO, NMC, LMO, LTO, solid-state), present limitations in lifespan and form factor. Tritium-powered batteries, with lifespans exceeding 20 years, enable more compact and versatile designs.
Challenge | Description |
|---|---|
Limitations of traditional batteries | Traditional chemical batteries, especially lithium-based, have a finite lifespan and size constraints. |
Power source reliability | Reliable power sources are critical for sustained device functionality. |
Impact on device design | Battery size affects the design and performance of implantable medical devices. |
1.2 Biocompatibility
Biocompatibility ensures that batteries do not trigger adverse reactions in the patient. Material selection plays a vital role in achieving this goal. Manufacturers use gelatin/polycaprolactone-based composite gel electrolytes in zinc ion batteries, which offer good biocompatibility and degradability. Conductive polymers and hydrogel electrolytes provide flexibility and compatibility for zinc-air batteries. Nanoporous gold serves as a catalytic cathode, while sodium-based alloys function as anodes, both demonstrating excellent biocompatibility. Zinc and magnesium-based alloys are also biodegradable and suitable for implantable applications.
Gelatin/polycaprolactone composite gel electrolyte
Conductive polymers for zinc-air batteries
Hydrogel electrolytes for flexibility
Nanoporous gold and sodium-based alloys
Zinc and magnesium-based biodegradable materials
1.3 Longevity
Longevity determines the replacement cycle and overall cost of implantable medical devices. Most batteries offer lifespans ranging from 5 to 25 years, depending on device type and usage. Implantable cardioverter-defibrillators typically last about 10.8 years, with some subtypes reaching up to 11 years. Factors such as manufacturer, time of implant, pacing mode, and pacing percentage influence battery life. Device size and number of shocks have minimal impact.
Factor | Impact on Longevity |
|---|---|
Manufacturer | Varies by brand |
Time of implant | Affects battery life |
Pacing mode | Influences energy consumption |
Pacing percentage | Higher usage reduces longevity |
Capacitor reformation interval | Affects battery performance |
Device size | No significant impact |
Number of shocks | No significant impact |
The selection of battery chemistry and design directly affects patient safety and device reliability. Engineers must balance miniaturization, biocompatibility, and longevity to meet the demands of modern implantable medical devices and their applications.
Part2: Miniaturization in Implantable Devices
2.1 Size Constraints
Batteries in implantable medical devices face significant size constraints. Engineers must develop compact, efficient, and safe power sources tailored to specific medical applications. The limited internal space of these devices restricts battery dimensions, which directly impacts the overall design and usability. For example, a bulky battery can make a wearable or implantable device impractical for daily use. Pediatric applications require even smaller batteries, while adult devices may allow for slightly larger cells. The form factor becomes a critical parameter early in the design process. Engineers must evaluate whether to use a user-accessible battery or a sealed rechargeable cell, always balancing capacity with compactness. This careful consideration ensures that implantable medical devices remain functional, ergonomic, and safe for patients.
Note: Size constraints not only affect the physical integration of the battery but also influence the selection of battery chemistries and the overall device architecture.
2.2 Energy Density
High energy density remains essential for implantable medical devices. These devices require batteries that deliver sufficient power over extended periods without frequent replacements or recharging. Engineers must select battery chemistries that maximize energy storage within the smallest possible volume. Lithium-based chemistries, such as LiFePO4, NMC, LCO, LMO, LTO, solid-state, and lithium metal, offer varying advantages in platform voltage, energy density, and cycle life. The table below compares these chemistries, highlighting their relevance for medical and other high-demand industries:
Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) | Application Scenarios |
|---|---|---|---|---|
LiFePO4 | 3.2 | 90-160 | 2000+ | Medical, Industrial |
NMC | 3.7 | 150-220 | 1000-2000 | Medical, Robotics, Security |
LCO | 3.7 | 150-200 | 500-1000 | Medical, Consumer Electronics |
LMO | 3.7 | 100-150 | 300-700 | Medical, Infrastructure |
LTO | 2.4 | 70-110 | 5000+ | Medical, Industrial |
Solid-state | 3.2-3.7 | 200-400 | 1000-2000 | Medical, Robotics |
Lithium metal | 3.0-3.6 | 300-500 | 500-1000 | Medical, Security |
Selecting the right chemistry depends on the device’s power requirements, expected lifespan, and safety profile. High energy density allows for longer operation in a smaller package, which is critical for implantable medical devices.
2.3 Device Integration
Integrating batteries with other components in implantable medical devices introduces several challenges:
Longevity of the device
Size miniaturization
Biocompatibility of materials
Safety regulations for commercialization
Slow advances in battery technologies
Need for novel materials and energy harvesting techniques
Engineers must address these challenges to ensure seamless operation and patient safety. Effective strategies for device integration include:
Power Management Techniques: Implementing energy-efficient components and dynamic power adjustment to optimize battery usage.
Material Selection for Battery Contacts: Choosing appropriate materials and designing contact interfaces to ensure reliable power delivery.
Design Considerations: Incorporating mechanisms that promote reliable contact and validating performance under physiological conditions.
Energy-efficient Components: Utilizing low-power microcontrollers and sensors to reduce power consumption.
Dynamic Power Adjustment: Adjusting power based on usage patterns to conserve energy during idle states.
Energy Harvesting Technologies: Implementing methods like piezoelectric energy harvesting to supplement battery power.
These strategies help optimize battery performance and extend device longevity. Engineers in the medical industry continue to innovate, seeking new materials and integration techniques to meet the evolving demands of implantable medical devices.
Part3: Biocompatibility and Safety

3.1 Material Selection
Material selection plays a critical role in the safety and performance of batteries for implantable medical devices. Engineers must choose biocompatible materials that do not cause harm or trigger adverse reactions in the body. Titanium, nanoporous gold, and sodium-based alloys are common choices because they resist corrosion and interact safely with biological tissues. Gelatin/polycaprolactone composite gel electrolytes and conductive polymers also offer excellent biocompatibility and flexibility, making them suitable for advanced applications. Zinc and magnesium-based alloys provide biodegradable options, which can reduce the need for surgical removal after the device’s service life ends.
Selecting the right materials ensures long-term biocompatibility and supports the device’s function throughout its lifespan. Manufacturers must also consider the source of raw materials to avoid ethical concerns. For more information on responsible sourcing, see the conflict minerals statement.
3.2 Immune Response
The human body can react to foreign objects, including implantable batteries, in several ways. Understanding these immune responses helps engineers design safer devices. The most common reactions include:
Promotion of dendritic cell maturation, which increases CD8 cytotoxic T cell and CD4 helper T cell activity.
Reduction in regulatory T cells (Tregs) and polarization of M2 macrophages to M1 macrophages, supporting adaptive immunity.
Generation of Zn2+ and Mn2+ ions, which can induce immunogenic cell death and activate the cGAS-STING pathway.
Increased secretion of type I interferon and proinflammatory cytokines, leading to greater T lymphocyte infiltration.
These processes can enhance the immune system’s response, but they may also cause inflammation or tissue damage if not properly managed. Engineers must select biocompatible materials and design features that minimize these risks, ensuring the safety of the patient and the reliability of implantable medical devices.
3.3 Regulatory Compliance
Strict regulatory standards govern the development and use of batteries in implantable medical devices. Compliance ensures that products meet safety and biocompatibility requirements before reaching the market. Key standards include:
Standard | Description |
|---|---|
Evaluates medical devices for potential adverse biological responses. | |
ISO 10993 | Provides guidance for evaluating biocompatibility, including cytotoxicity and sensitization. |
IEC 62133 | Sets safety requirements for batteries used in medical devices. |
UL 2054 | Ensures batteries are biocompatible and safe for use. |
ISO 13485 | Defines quality management systems for medical devices, supporting biocompatibility. |
IEC 60601-1 | Covers basic safety and essential performance of medical electrical equipment. |
Regulatory requirements can differ by region. For example:
Region | Regulatory Body | Key Standards and Requirements |
|---|---|---|
United States | FDA | IEC 62133, IEC 60086-4, UL 1642, UL 2054, among others for battery safety and performance. |
Europe | MDR | ANSI/AAMI ES 60601-1, IEC 60086-4, IEC 62133, which include safety and performance testing for medical devices. |
Transportation | Various Agencies | UN 38.3 testing requirements for safe transport of lithium batteries, including altitude simulation and thermal tests. |
Manufacturers must demonstrate that their batteries meet these standards through rigorous testing. The ISO 10993 series, for example, covers cytotoxicity, sensitization, irritation, and genotoxicity, ensuring that implantable medical devices are safe for human use. Compliance with these regulations protects both the patient and the manufacturer, supporting the safe use of lithium battery packs in medical, industrial, and other high-demand applications.
Part4: Power Management and Stability
4.1 Battery Life
Maximizing battery life remains a top priority for engineers working with batteries for implantable medical devices. Device longevity directly impacts patient safety and healthcare costs. The latest lithium manganese dioxide batteries deliver up to 1.9 amp-hours of usable capacity, setting an industry benchmark. These batteries support devices that last up to 13.2 years, reducing the need for frequent replacements. The table below highlights key features that contribute to extended battery life:
Feature | Details |
|---|---|
Capacity | 1.9 Amp-hours of usable battery capacity – highest in the industry |
Chemistry | Lithium manganese dioxide maintains voltage and resistance stability |
Efficiency | Up to 8% smaller and 24% thinner devices |
Works for up to 13.2 years, outlasting the competition | |
Cost Savings | Fewer replacements reduce costs for patients and healthcare systems |
Clinically proven since 2008 with impressive longevity |
Engineers also explore self-powered solutions using energy harvesting technologies. These include electromagnetic energy harvesting, ultrasound wireless power transfer, and thermoelectric generators that utilize body heat. Such innovations further extend device life and reliability.
4.2 Power Output
Implantable medical devices require stable and predictable power output to function safely. Different devices have unique power needs, from low-power sensors to high-output stimulators. The table below compares common energy sources and their generated power:
Energy Harvesting Method | Approaches | Generated Power | Advantages | Disadvantages |
|---|---|---|---|---|
Independent system | Lithium batteries | Compatibility with flexible electronic | Size | |
Bio-fuel cells | 2.4 μW | Recycle materials | Low output power | |
Nuclear batteries | 50 μW | Longer service life (>15 years) | Radioactive danger | |
Thermoelectricity | 5.8 µW | Unlimited lifetime | Low output power | |
Piezoelectricity | 2.1–69.8 W | High output power | Limited implantable locations |
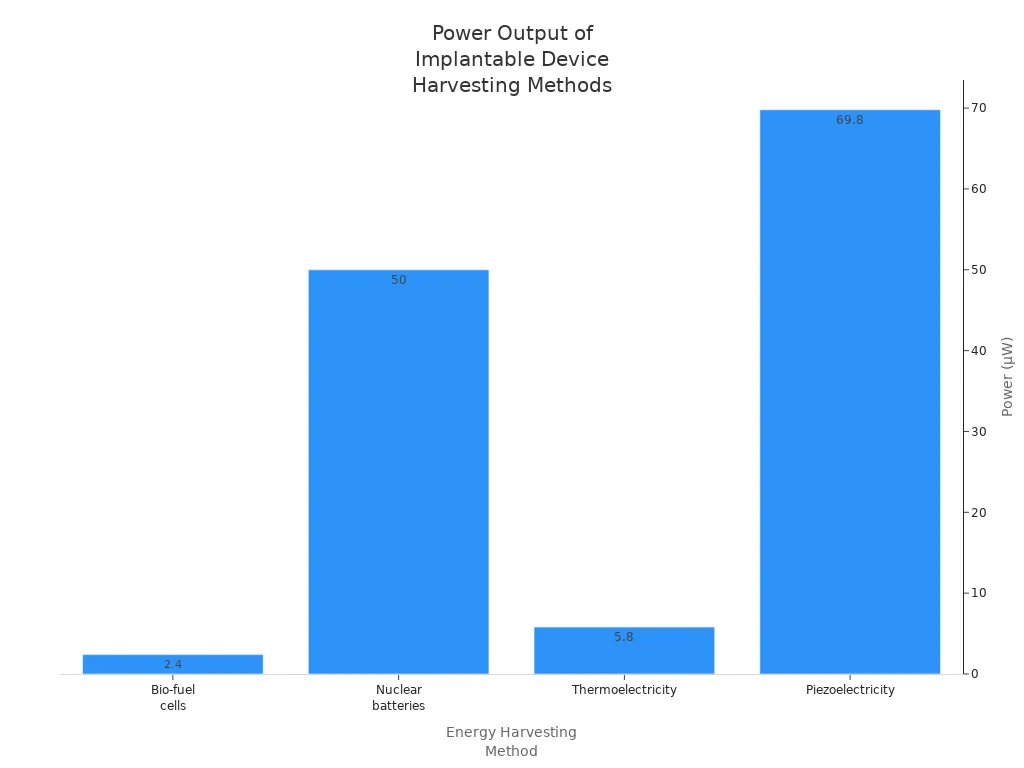
Stable power output ensures device reliability and patient safety. Engineers select the appropriate chemistry and energy harvesting method based on the device’s application, such as medical, robotics, or security.
4.3 Long-Term Performance
Long-term performance depends on several factors, including battery chemistry, device type, and energy consumption. The internal design of the battery, such as the stacked plate structure in ENDURALIFE batteries, maximizes power capacity and energy density. Li/MnO2 batteries maintain a voltage above 2.8 V and stable internal resistance, supporting a higher elective replacement indicator. In contrast, Li/SVO batteries show a voltage drop and increased resistance as they deplete.
The main factors affecting the long-term performance of implantable batteries include the device manufacturer, device type (ICD vs. CRT-D), and the ventricular pacing rate. Additionally, the energy consumed by the device and the energy available from the battery are critical factors. The chemistry and internal design of the battery also play significant roles in determining battery longevity.
To further enhance reliability, engineers implement advanced battery management systems. These systems monitor battery health, optimize charging cycles, and prevent over-discharge.
Innovations in energy harvesting technologies, such as glucose oxidation within biofuel cells and energy generation from tissue motion, continue to improve the long-term stability of implantable devices. These advancements help ensure that implantable medical devices remain safe and effective throughout their service life.
Part5: Innovations in Implantable Battery Technology
5.1 Advanced Chemistries
Recent years have seen a shift from outdated battery chemistries to advanced lithium-based solutions, such as LiFePO4, NMC, LCO, LMO, LTO, solid-state, and lithium metal. These chemistries offer higher energy density, longer cycle life, and improved safety profiles, making them ideal for medical, robotics, and security applications. Engineers now explore battery-free bioelectronic implants that harvest energy directly from the body. This approach eliminates bulky batteries and reduces device size. Nanogenerator technologies, including biofuel cells that generate electricity from glucose and thermoelectric harvesting from temperature gradients, have gained traction. Triboelectric generators, made from flexible and biodegradable materials, enable energy harvesting from body movements. These advancements support both miniaturization and biocompatibility, which remain critical for implantable medical devices.
Battery-free bioelectronic implants harvest energy from the body
Nanogenerators use glucose or temperature gradients for power
Triboelectric generators leverage body movements and flexible materials
5.2 Manufacturing Techniques
Manufacturers have adopted new techniques to improve the reliability and safety of implantable batteries. One notable advancement is the use of a novel electrolyte called a catholyte. This innovation combines the functions of the cathode and electrolyte, reducing overall battery weight. The new catholyte enhances battery lifetime by up to 50% or allows for smaller, lighter batteries without increasing costs. Safety improves as these cells avoid toxic and corrosive materials found in older chemistries. Preliminary tests show a stable shelf life exceeding one year, which is essential for primary batteries in medical devices. These improvements help ensure consistent performance and patient safety.
Catholyte electrolytes reduce weight and increase battery life
Safer cells avoid toxic and corrosive materials
Stable shelf life supports long-term device reliability
5.3 Industry Collaboration
Cross-industry partnerships drive innovation in implantable battery technology. Medical device manufacturers, battery specialists, and materials scientists work together to develop solutions that meet strict regulatory and performance standards. Collaboration accelerates the adoption of advanced chemistries and manufacturing methods. It also fosters the integration of energy-harvesting technologies into next-generation devices. These partnerships ensure that new batteries address the unique demands of medical, industrial, and security sectors. Companies that prioritize sustainability in their supply chains further enhance their reputation and compliance. For more on sustainable practices in battery manufacturing, see our approach to sustainability.
Ongoing innovation in batteries for implantable medical devices remains essential for advancing patient care and device reliability. Miniaturization, biocompatibility, and regulatory compliance drive progress in the field. Industry experts expect several trends to shape the next decade:
Advancements in solid-state batteries will improve safety and performance.
Wireless charging systems will reduce surgical interventions.
Sustainability will increase with biodegradable and recyclable technologies.
Stricter regulations will encourage safer, eco-friendly solutions.
Market growth will accelerate as technology and consumer needs evolve.
FAQ
What are the main battery chemistries used in implantable medical devices?
Engineers use LiFePO4, NMC, LCO, LMO, LTO, solid-state, and lithium metal chemistries. These options offer high energy density, stable platform voltage, and long cycle life. Selection depends on device requirements in medical, robotics, or security applications.
How do size constraints impact lithium battery pack design for implants?
Size constraints require engineers to develop compact lithium battery packs. Smaller form factors must still deliver high energy density and reliability. This challenge drives innovation in both chemistry and packaging for medical and industrial devices.
Why is biocompatibility critical for implantable lithium batteries?
Biocompatibility ensures that battery materials do not trigger immune responses or tissue damage. Manufacturers select materials like titanium and nanoporous gold to meet strict medical standards and regulatory requirements, protecting patient safety and device performance.
What regulatory standards apply to lithium batteries in medical devices?
Manufacturers must comply with FDA guidelines, ISO 10993, IEC 62133, and UL 2054. These standards address safety, biocompatibility, and quality management for lithium battery packs in medical, industrial, and security sectors.
How do engineers maximize battery life in implantable devices?
Engineers select advanced chemistries, optimize power management, and use energy harvesting technologies. These strategies extend battery life, reduce replacement frequency, and improve reliability for medical and industrial applications.




