You face strict demands when designing battery systems for medical devices. Lithium battery packs help you achieve compliance and enhance safety. Failing to meet these standards can delay certification and block access to regulated markets. Careful design choices protect your reputation and support reliable performance.
Key Takeaways
Understand IEC 60601-1 standards to ensure safety and compliance in medical device battery systems. This knowledge helps avoid costly redesigns and regulatory delays.
Implement robust safety measures, such as Battery Management Systems and thermal management strategies, to mitigate risks associated with lithium battery packs.
Maintain thorough documentation throughout the design process. This practice supports compliance and streamlines certification for medical devices.
Part 1: 60601 Overview
1.1 Standard Scope
You must understand the broad reach of IEC 60601-1 when designing battery systems for medical devices. This standard applies to all medical electrical equipment and systems, including those powered by lithium battery packs. You will find that IEC 60601-1 covers devices powered by a single power cord, hard-wired connections, or batteries. Many medical devices rely on battery operation, such as thermometers, gamma imaging systems, endoscopic cameras, and infusion pumps.
IEC 60601-1 sets requirements for insulation, leakage current, and risk management. These requirements help you address electrical and mechanical safety, which are critical for patient protection and device reliability. You must consider these factors early in your design process to meet compliance and avoid costly redesigns.
1.2 Key Compliance Goals
You need to focus on the primary goals of 60601 compliance. The standard aims to ensure patient safety by minimizing the risk of electrical hazards, such as shocks or burns. You must also meet strict requirements for insulation and leakage current to protect both patients and operators.
60601 standards require you to manage risk through robust design and thorough testing.
You must document your compliance efforts to support regulatory approval and certification.
Essential performance and safety standards guide your design choices and testing protocols.
IEC 60601 compliance is critical for medical device compliance and market approval. You will face regulatory scrutiny if you do not meet these compliance requirements. By prioritizing safety, performance, and thorough documentation, you can streamline certification and ensure your devices reach the market efficiently.
Part 2: Safety in Designing Battery Systems

2.1 Electrical Hazards
You must address electrical hazards as a top priority when designing battery systems for medical devices. Lithium battery packs present unique risks, including overcurrent, short circuits, and thermal runaway. To meet 60601 requirements, you need to implement robust strategies that protect both patients and equipment.
Strategy Type | Description |
|---|---|
Safety Devices | Incorporate protection vents, current fuses, and additives to prevent excessive current flow. |
Thermal Management | Use air cooling, liquid cooling, or phase change cooling to manage heat accumulation. |
Battery Management Systems | Monitor operations, detect unsafe conditions, and manage thermal effects and fault diagnostics. |
You should select certified components and integrate a reliable Battery Management System (BMS) to ensure continuous monitoring and control. This approach supports compliance with 60601 standards and reduces the risk of electrical failure. Proper thermal management also prevents overheating, which can lead to dangerous situations in medical environments.
2.2 Mechanical Hazards
Mechanical hazards can compromise both safety and essential performance in medical electrical equipment. You must evaluate risks related to instability, drops, vibration, and moisture exposure. 60601 compliance requires you to test for these hazards and modify your design to ensure functional integrity.
Test for instability, especially in mobile medical devices.
Focus on mechanical strength, prioritizing basic safety and essential performance rather than just avoiding unacceptable risk.
Adhere to IEC 60086-4 and IEC 62133 standards for lithium battery packs.
To prevent mechanical failures, you should:
Charge lithium-ion and lithium-polymer batteries using a multistate charge profile for safety and longevity.
Follow the correct voltage and current sequence during charging.
Implement mechanical protection against drops, vibration, and moisture.
Analyze user behavior and potential misuse to design effective safeguards.
Use a Protection Circuit Module (PCM) for each cell to limit charge/discharge voltages and currents, preventing thermal instability.
These practices help you meet regulatory approval and maintain high performance in demanding medical and industrial environments.
2.3 Insulation & Leakage
Insulation and leakage current requirements form the backbone of 60601 safety standards. You must understand the insulation classes and leakage limits for different types of medical devices.
IEC 60601-1 outlines insulation requirements for all medical electrical equipment, including battery systems.
Class I devices require protective earthing and basic insulation.
Class II devices use double or reinforced insulation without protective earthing.
Patient leakage current and auxiliary current limits apply to all applied parts.
Risk management may identify additional parts that must meet applied part requirements.
Certified low-leakage power solutions play a critical role in achieving compliance. The table below summarizes leakage current limits for different device types:
Type | Description | Leakage Current Limits (Normal/Single Fault) |
|---|---|---|
B | Devices with no direct patient contact | 100 µA / 500 µA |
BF | Body floating devices | 100 µA / 500 µA |
CF | Cardiac floating devices | 10 µA / 50 µA |
You can also visualize the differences in leakage current requirements:
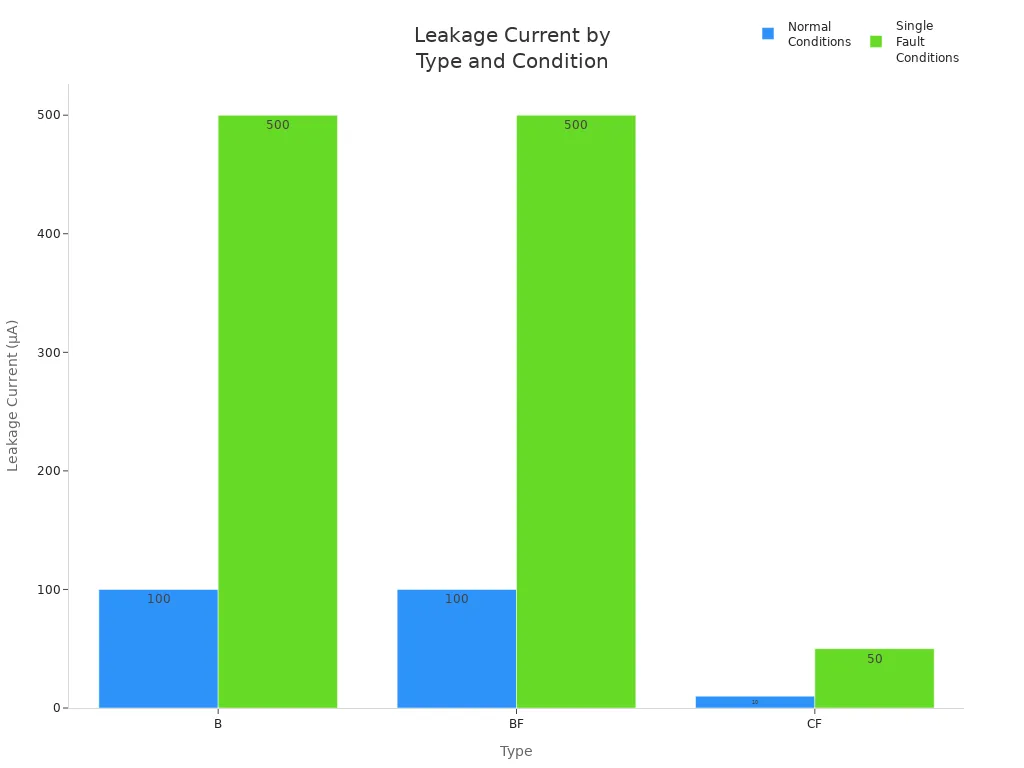
By selecting certified, low-leakage solutions and following strict insulation protocols, you ensure your battery systems meet 60601 and IEC standards. This approach supports both safety and essential performance, helping you achieve certification and streamline testing and validation.
Part 3: Risk Management & Compliance
3.1 Hazard Analysis
You must start your risk management process with a thorough hazard analysis. IEC 60601 and ISO 14971 require you to identify and evaluate all potential hazards associated with lithium battery packs in medical devices. This step ensures you address both safety and essential performance from the beginning of your design.
The most frequently identified hazards in battery-powered medical devices include:
Hazard Type | Description |
|---|---|
Thermal Runaway | A condition where a battery overheats, potentially leading to fires and explosions. |
Improper Management | Risks from inadequate battery management practices, which can cause failures and downtime. |
Unapproved Components | Use of non-approved batteries or chargers that may not meet safety standards, increasing risk. |
Toxic Gas Release | Lithium-ion battery failures can emit harmful gases and materials, posing health risks. |
You need to recognize that thermal runaway can cause bodily injury and property damage. Poor battery management increases the risk of device failure, which can compromise patient care. Using only approved components and chargers helps you meet safety standards and reduce hazards. Design improvements and robust management practices further mitigate these risks.
Tip: Understanding how your medical devices interact with lithium battery packs is essential for accurate risk assessment and reliable performance.
3.2 Risk Controls
After identifying hazards, you must implement risk control measures that align with IEC 60601 and ISO 14971 requirements. These standards direct you to prioritize inherent safety measures in your design. You should always document your risk control strategies and their effectiveness in your risk management report.
Recommended risk control measures include:
Use high-safety components and functionally safe architecture.
Integrate established software libraries for battery management.
Install backup batteries to maintain device functionality during power loss.
Conduct verification and validation of all risk-minimizing measures.
Apply risk controls throughout the product life cycle, including production and operation.
You must keep risks at an acceptable level, especially if a critical function is lost. For example, a backup battery can prevent loss of essential performance during a power outage. Regular safety testing and validation confirm that your controls work as intended.
Note: AAMI surveys show that battery management remains a top challenge for healthcare technology professionals. Poor management can lead to device failures, increased costs, and compromised patient safety. You can reduce these risks by implementing design modifications, additional controls, and user training.
3.3 Documentation
Thorough documentation forms the backbone of your compliance strategy. You must map all design requirements to your compliance documentation to satisfy IEC 60601 and regulatory standards. Early engagement with certification bodies helps you clarify expectations and avoid costly redesigns.
Best practices for documentation include:
Systematic documentation management from the start of your project.
Regular compliance reviews throughout development.
Comprehensive technical specifications for all lithium battery systems.
Detailed testing evidence, including safety testing and validation results.
Robust quality management systems to support ongoing compliance.
You should maintain clear records of all risk assessments, control measures, and testing requirements. This approach streamlines certification and supports regulatory approval for your medical devices. Mapping your design requirements to documentation ensures you meet all 60601 and IEC standards, reducing the risk of delays during certification.
Tip: Early and systematic documentation not only supports compliance but also strengthens your position in regulated markets.
Part 4: Testing for IEC 60601 Compliance

4.1 Electrical Tests
You must conduct thorough electrical testing to meet 60601 requirements for medical devices. These tests verify that your lithium battery packs operate safely and reliably under all expected conditions. You will measure insulation resistance, leakage current, and dielectric strength. Testing also includes verifying overcurrent protection and short-circuit response. These steps ensure your systems meet IEC safety standards and support essential performance. Electrical testing forms the foundation of compliance and helps you identify design issues early.
4.2 Environmental Tests
Environmental testing evaluates how your battery systems perform in real-world medical settings. You will expose devices to temperature extremes, humidity, vibration, and mechanical shock. These tests confirm that your design maintains safety and performance even in harsh environments. IEC 60601 compliance requires you to simulate conditions such as accidental drops or exposure to fluids. By following these requirements, you reduce risk and support regulatory approval for your devices.
4.3 Verification Steps
A systematic approach to testing and validation helps you avoid last-minute changes and delays. You should follow a structured process from initial design to final testing. The table below outlines recommended steps for verifying compliance with IEC 60601 standards:
Step | Description |
|---|---|
1 | Review component information to ensure compliance with standards. |
2 | Update isolation diagram to reflect the latest design. |
3 | Ensure the test plan is current and comprehensive. |
4 | Verify that marking and labeling meet the required standards. |
5 | Finalize Risk Management Framework (RMF) and essential performance. |
6 | Conduct pre-testing of the device to identify potential issues. |
7 | Prepare necessary documentation and materials for testing. |
8 | Maintain communication with test labs throughout the process. |
Tip: Early and systematic testing, combined with clear documentation, streamlines compliance and supports successful regulatory submissions.
You achieve compliance by selecting certified power supplies, increasing isolation barriers, and following patient connection guidelines. Early risk management and systematic testing help you meet requirements and improve safety and efficacy. Collaborate with regulatory experts and maintain thorough documentation to keep your lithium battery packs aligned with evolving standards.
FAQ
What makes lithium battery packs suitable for the medical device industry?
You benefit from high energy density, long cycle life, and stable platform voltage. These features support reliable operation in medical, robotics, and industrial applications.
How do essential performance clauses affect battery system design?
You must ensure lithium battery packs maintain critical functions under fault conditions. Essential performance clauses guide your risk controls and testing protocols.
Can Large Power provide custom lithium battery solutions for medical devices?
You can request a custom consultation from Large Power for tailored lithium battery packs. Contact Large Power for custom battery solutions.




