You rely on a medical-grade battery pack to power critical medical devices where patient safety comes first. High standards for safety and reliability set these batteries apart from consumer models. Regulatory compliance remains vital because failures can cause serious incidents.
Incident Type | Description |
|---|---|
Fires | Lithium-ion batteries can catch fire due to thermal runaway, leading to severe consequences. |
Explosions | Explosions can occur from thermal runaway, causing unique damage classified as a separate safety concern. |
Leaks | Battery leaks can expose users to harmful substances. |
Off-gassing | Toxic gases can be released during battery failure, posing health risks. |
Key Takeaways
Medical-grade battery packs prioritize safety and reliability, meeting strict regulatory standards to protect patient health.
Key components like the Battery Management System (BMS) monitor performance, preventing overcharging and ensuring safe operation.
Lithium-ion batteries dominate the medical market due to their high energy density and long cycle life, making them ideal for critical devices.
Always verify that battery packs include essential safety features like thermal shutdown and overcharge protection to minimize risks.
Regular maintenance and proper storage of batteries can significantly extend their lifespan and ensure consistent performance in medical devices.
Part1: Medical-Grade Battery Pack Structure

1.1 Core Components
You encounter several critical components when you examine a medical-grade battery pack. Each part plays a distinct role in powering medical devices safely and reliably. The main physical and electrical components include:
Component Type | Description |
|---|---|
Anode | Stores and releases electrons during charge and discharge cycles. |
Cathode | Receives electrons and determines the battery’s voltage and capacity. |
Separator | Prevents direct contact between anode and cathode, reducing the risk of short circuits. |
Electrolyte | Enables ion movement between electrodes, supporting energy transfer. |
Current Collectors | Conduct electricity from electrodes to external circuits. |
Casing | Encases all components, providing mechanical protection and insulation. |
Voltage Configuration | Series-connected cells increase voltage; parallel connections boost capacity. |
Capacity Specification | Nominal capacity (Ah) indicates how much charge the medical battery can store and deliver. |
Protection Circuit Module | Monitors cell voltages and controls charge/discharge limits for safety. |
Smart Protection Systems | Combine electrical and physical safety features to prevent overcharging and discharging. |
You see these components working together to deliver consistent power and maintain safety in demanding healthcare environments. The medical battery market demands strict adherence to these design principles to ensure reliability.
1.2 Protective Casing and Design
The casing of a medical-grade battery pack serves as the first line of defense against physical damage and chemical hazards. You often find fire-rated, injection-molded plastic used for user-accessible batteries. This material offers self-extinguishing properties and meets the UL94-5 safety rating. Shrink-wrap plastic appears in non-accessible batteries but does not provide reliable fire protection.
Material Type | Description | Safety Rating |
|---|---|---|
Fire-rated, injection-molded plastic | Used for user-accessible batteries; self-extinguishing properties enhance safety. | UL94-5 |
Shrink-wrap plastic | Used for non-accessible batteries; not reliable for fire protection. | N/A |
You must consider environmental controls in medical settings. Static control flooring, footwear, and mobile equipment help dissipate static charges in operating rooms. Equipment grounding ensures all medical devices remain safe from electrostatic discharge. Low-charging and dissipative materials minimize risks, while ionization neutralizes static charges in the air and on insulative items. Maintaining humidity levels between 40% and 60% further reduces static electricity and associated hazards.
Tip: Always verify that the casing material and design meet the required safety ratings and environmental controls for your application.
1.3 Battery Management System
A battery management system (BMS) acts as the brain of your medical-grade battery pack. You rely on the BMS to monitor temperature, voltage, state of health (SOH), and state of charge (SOC) for each cell. The BMS protects against overcharging, over-discharging, short circuits, and thermal runaway. It continuously manages voltage, current, temperature, and SOC, preventing dangerous conditions and maximizing battery life.
Monitors temperature, voltage, SOH, and SOC for each cell.
Protects against overcharging, over-discharging, short circuits, and thermal runaway.
Maximizes battery life and ensures safe operation in medical devices.
You can learn more about BMS and its role in lithium battery packs by visiting this resource.
Design choices in the BMS and casing directly impact the safety and reliability of medical batteries. You must select systems that meet the rigorous demands of healthcare environments, where uninterrupted power and patient safety are non-negotiable.
Part2: Medical Battery Chemistries

2.1 Lithium-Ion in Medical Devices
You see lithium-ion batteries dominate the medical device market due to their high energy density, lightweight design, and long cycle life. These batteries power a wide range of critical equipment, from portable diagnostic tools to implantable devices. Their reliability and longevity make them the preferred choice for healthcare applications, where uninterrupted performance is essential. The lithium-ion medical battery market continues to grow, driven by the need for safe and efficient power sources in demanding environments.
Key advantages of lithium-ion battery chemistry include:
High energy density (150–250 Wh/kg), enabling compact and portable device designs.
Long cycle life, supporting up to 1,000 full charge cycles.
Low self-discharge rate, losing only 2–3% charge per month.
Stable voltage output, rapid charging, and wide temperature tolerance.
You benefit from these features in medical, robotics, security systems, infrastructure, consumer electronics, and industrial sectors. The table below compares standardized lithium battery chemistries used in these industries:
Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) | Typical Applications |
|---|---|---|---|---|
NMC | 3.7 | 150–220 | 1,000–2,000 | Medical, robotics, security, infrastructure |
LCO | 3.7 | 150–200 | 500–1,000 | Consumer electronics, medical |
LMO | 3.7 | 100–150 | 300–700 | Medical, industrial, power tools |
LTO | 2.4 | 70–80 | 5,000–10,000 | Industrial, infrastructure, medical |
LiFePO4 | 3.2 | 90–140 | 2,000–5,000 | Medical, robotics, security, infrastructure |
Note: For more on sustainability in lithium battery chemistry, see our approach to sustainability.
2.2 Alternative Chemistries
While lithium-ion remains the standard, you may encounter alternative battery chemistry options for specific medical applications. Nickel-metal hydride and lithium-manganese dioxide serve in devices with lower power needs or where stability is critical. The table below outlines common chemistries and their typical uses:
Battery Chemistry | Typical Applications |
|---|---|
Lithium Ion Batteries | Widely used in various medical devices |
Lithium-Manganese Dioxide (LiMnO2) | Powers defibrillators like the Philips HeartStart |
Lithium-Carbon Fluorides (Li-(CFx)) | Used in interventional and implantable medical devices |
Lithium Iron Phosphate (LiFePO4) | Found in portable medical devices and surgical tools |
Nickel Metal Hydride | Powers small rechargeable medical devices |
Nickel Cadmium | Used in blood pressure monitors and diabetic monitors |
Alkaline Batteries | Common in BP monitors, pulse oximeters, and infusion pumps |
Zinc Air Batteries | Primarily used in hearing aids |
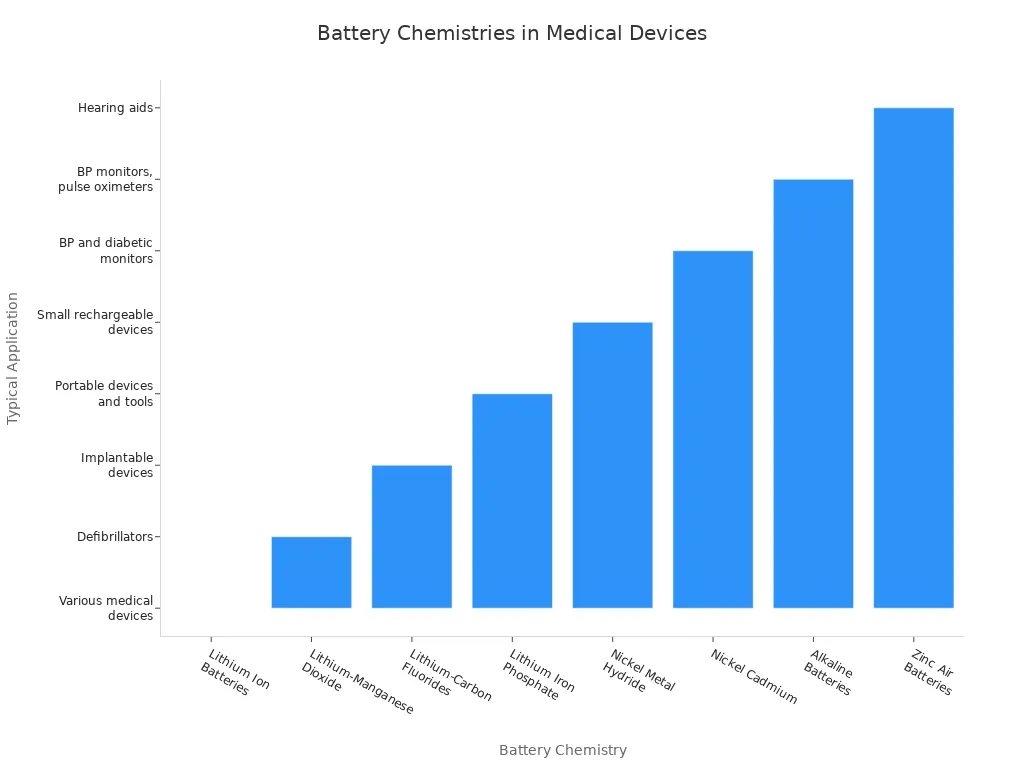
You should note that nickel-metal hydride batteries offer stable performance and lower risk of thermal runaway, but they have lower energy density and moderate cycle life compared to lithium-ion. Lithium polymer batteries, while similar to lithium-ion, provide flexible form factors but require careful containment.
For information on conflict minerals in battery chemistry, review our conflict minerals statement.
2.3 Chemistry Selection Criteria
You must evaluate several factors when selecting the right battery chemistry for your medical device. Manufacturers consider energy requirements, peak current, safety certifications, and regulatory compliance. The table below summarizes key criteria:
Criteria | Description |
|---|---|
Required Battery Energy | Total current needs for the device’s architecture. |
Peak Current | Maximum current during operation. |
Safety Certifications | Compliance with IEC 62133 and other standards. |
Regulatory Standards | Adherence to medical device regulations. |
Operational Characteristics | Continuous or intermittent use, expected lifespan. |
Protection against environmental factors. | |
Cell Size | Physical dimensions of the battery. |
Cost | |
Availability | Market supply of the chemistry. |
Safety Issues | Potential hazards unique to each chemistry. |
Replacement Schedule | Planning for cell replacement and cycle life. |
Energy Capacity Design Margin | Ensuring sufficient capacity for operational demands. |
Shipping Restrictions | Limitations on transporting certain chemistries. |
Risk of Mixing Cells | Dangers of mixing different chemistries during replacement. |
Battery chemistry selection directly impacts safety and regulatory compliance. Parameters such as charge capacity, charge density, discharge currents, and operating temperature influence both device performance and adherence to standards. You must ensure your choice aligns with operational needs and industry regulations.
Part3: Safety and Regulatory Standards
3.1 Built-In Safety Features
You must prioritize safety when designing or sourcing a medical-grade battery pack. International safety standards require you to integrate multiple protective features into every medical battery. These features help prevent failures that could endanger patients or disrupt critical medical devices.
Safety Feature | Description |
|---|---|
IEC 62133 | Sets safety requirements for batteries used in medical devices. |
UL 2054 | Defines safety standards for battery packs. |
ISO 13485 | Focuses on quality management for medical devices. |
IEC 60601-1 | Covers general safety for medical electrical equipment. |
Biocompatibility | Ensures materials are safe for patient contact. |
Authentication | Prevents use of counterfeit batteries. |
Serialization | Enables traceability for each battery pack. |
Overcharge Protection | Stops charging when voltage exceeds safe limits. |
Thermal Shutdown | Activates if the battery overheats. |
You rely on these features to protect against overcharging, overheating, and counterfeit risks. Overcharge protection and thermal shutdown circuits act as your first defense against electrical faults. Serialization and authentication help you track each medical battery throughout its lifecycle, supporting recalls and quality control.
Thermal cutoffs and current interrupt devices (CIDs) play a vital role in preventing battery-related incidents. These components manage gas generation and pressure relief during thermal runaway events. For example, the addition of a second vent in the 18650 cell design improved pressure relief and reduced rupture risk. You benefit from these design improvements, which lower the chance of catastrophic failure in medical devices.
Evidence Description | Implication for Safety Devices |
|---|---|
Integrated safety devices like CIDs and thermal cutoffs manage gas and pressure. | These devices are critical for mitigating battery failure risks. |
A second vent in 18650 cells improved pressure relief and reduced rupture risk. | Enhanced design features improve safety outcomes. |
⚠️ Tip: Always verify that your medical-grade battery pack includes these built-in safety features and meets all relevant safety standards.
3.2 Regulatory Compliance (ANSI/AAMI ES 60601-1)
You must ensure that every medical battery meets strict regulatory requirements. ANSI/AAMI ES 60601-1 stands as the primary safety standard for battery-powered medical devices in the United States and many global markets. This standard outlines essential safety and performance criteria that you must follow.
Requirement | Description |
|---|---|
General Safety | Sets basic safety and performance for devices using electrical outlets or batteries. |
Risk Management | Requires a risk management model to assess device safety. |
Essential Performance | Defines performance metrics to protect users and patients. |
Compliance for Lithium Batteries | Demands adherence to IEC 60086-4 or IEC 62133 for safe lithium battery operation. |
You need to document your risk management process and demonstrate that your medical battery meets all essential performance requirements. Compliance with IEC 62133 or IEC 60086-4 ensures that your lithium battery packs operate safely under both normal and foreseeable misuse conditions. Regulatory requirements like these help you minimize hazards and maintain trust with healthcare providers.
📋 Note: Regulatory compliance is not optional. You must meet these safety standards to market your medical devices legally and protect patient safety.
3.3 Testing and Certification
You cannot overlook the importance of rigorous testing and certification for any medical-grade battery pack. Testing verifies that your medical battery meets all safety standards and regulatory requirements before it enters the market.
Standard/Agency | Description |
|---|---|
UL2054 | Standard for battery packs in medical devices. |
IEC 62133 | Optional certification for lithium batteries. |
UL 1642 | Optional certification for lithium batteries. |
Required in Europe for safety and EMC compliance. | |
Agencies | UL, CSA, SGS, Intertek conduct testing and certification. |
You must submit your medical battery to accredited labs for evaluation. These agencies test for electrical safety, thermal stability, and compliance with electromagnetic compatibility (EMC) standards. Passing these tests allows you to display certification marks like UL or CE, which signal to buyers and regulators that your medical devices meet the highest safety standards.
✅ Alert: Always choose battery suppliers who provide full documentation of testing and certification. This step protects your business and ensures patient safety.
Part4: Performance and Reliability in Medical Devices
4.1 Lifespan and Cycle Life
You depend on medical-grade battery packs to deliver consistent battery performance throughout the lifespan of your medical devices. Manufacturers design these batteries for extended service life, far surpassing consumer-grade alternatives. The following table highlights the typical lifespan and cycle life for various battery types used in medical devices:
Battery Type | Lifespan | Cycle Life |
|---|---|---|
Medical Li-ion | Up to 20 years | Up to 5,000 cycles |
Consumer Li-ion | Up to 5 years | 500 to 1,000 cycles |
Nickel-metal hydride | 3-5 years | 500 to 1,000 cycles |
Alkaline | 1-2 years | N/A |
Silver-oxide | 2-5 years | N/A |
Zinc-air | 6-12 months | N/A |
You notice that medical lithium-ion battery packs offer up to 20 years of reliable battery performance and can withstand up to 5,000 charge cycles. This durability ensures your medical devices remain operational in demanding healthcare environments.
Tip: Always verify the expected cycle life and lifespan when selecting batteries for your medical devices.
4.2 Reliability Under Stress
You require battery performance that remains stable under stress conditions. Medical-grade battery packs undergo rigorous testing to ensure reliability during fires, explosions, and exposure to toxic gases. These batteries withstand extrinsic triggers such as overcharging, temperature spikes, and mechanical impacts. Intrinsic triggers like internal short-circuiting are also addressed through advanced design.
Manufacturers test battery packs at extreme temperatures, from -40° to 85°C, and under high-load scenarios. Medical lithium-ion battery packs, including the TLM Series, deliver high voltage and instant activation, supporting continuous loads up to 5A and pulses up to 15A. These features guarantee that your medical devices maintain battery performance even in critical situations.
Safety events: fires, explosions, toxic gas release, and liquid leakage.
Stress triggers: overcharging, temperature spikes, mechanical impacts, and internal short-circuiting.
Medical lithium-ion packs: reliable operation in extreme temperatures and high-load applications.
4.3 Maintenance and Serviceability
You must implement strict maintenance protocols to preserve battery performance and maximize device uptime. Routine testing and calibration during preventive maintenance extend battery life and reduce costs. Manufacturers recommend storing batteries in cool, dry environments, maintaining a 50% charge for long-term storage, and avoiding extreme temperatures.
Regularly check and recharge batteries.
Perform occasional charging cycles, even when not in use.
Handle batteries carefully and use protective covers.
Store in non-conductive containers with proper ventilation.
Serviceability plays a crucial role in healthcare settings. Swappable battery packs enable zero downtime, ensuring your medical devices remain operational and ready for use. Reliable battery solutions enhance efficiency and reduce interruptions in patient care.
You need medical-grade battery packs that deliver safety, reliability, and compliance for medical devices. The table below highlights the features that set these battery packs apart:
Feature | Description |
|---|---|
Safety Standards | Medical batteries must adhere to strict safety measures to protect users. |
Regulatory Compliance | Compliance with medical regulations is essential for safety and efficacy. |
Performance | Designed for long-lasting power and reliability in medical devices. |
Cycle Life | High cycle life supports demanding medical applications. |
Shelf Life | Long shelf life ensures readiness for medical devices. |
Self-discharge Rate | Low self-discharge maintains charge for critical medical devices. |
You should focus on careful component selection, robust chemistry, and strict regulatory compliance. These steps protect patient safety and ensure your medical devices perform as expected. When sourcing battery solutions, consider capacity, discharge rate, cycle life, safety features, and customization options. Industry leaders use in-house testing, ISO-certified processes, and advanced safety analysis to support the reliability of medical devices. By prioritizing these factors, you help guarantee the safety and performance of every medical device you deliver.
FAQ
What makes a battery pack “medical-grade”?
You find medical-grade battery packs designed for strict safety, reliability, and regulatory compliance. Manufacturers use advanced lithium-ion chemistries, robust casing, and integrated safety systems. These packs meet standards such as IEC 62133 and ANSI/AAMI ES 60601-1 for healthcare environments.
How do lithium-ion battery packs compare to nickel-metal hydride in medical devices?
Chemistry | Platform Voltage (V) | Energy Density (Wh/kg) | Cycle Life (cycles) | Typical Medical Use |
|---|---|---|---|---|
Lithium-Ion | 3.7 | 150–250 | 1,000–5,000 | Portable monitors, pumps |
Nickel-Metal Hydride | 1.2 | 60–120 | 500–1,000 | Small rechargeable devices |
You gain higher energy density and longer cycle life with lithium-ion packs.
What safety features should you look for in a lithium battery pack for medical devices?
You should confirm the pack includes overcharge protection, thermal shutdown, serialization, and authentication. Integrated Battery Management Systems (BMS) monitor temperature, voltage, and current. These features help prevent fires, explosions, and counterfeit risks.
How often should you replace medical-grade lithium battery packs?
You should follow manufacturer guidelines. Most medical lithium-ion battery packs last up to 20 years or 5,000 cycles. Regular maintenance and testing help you maximize lifespan and ensure reliable device operation.
Which industries use medical-grade lithium battery packs?
You see medical-grade lithium battery packs in healthcare, robotics, security systems, and infrastructure. These packs power portable diagnostic tools, infusion pumps, surgical equipment, and emergency devices. Reliable performance supports critical operations in demanding environments.




