
Medical grade rechargeable batteries provide critical power for medical devices, ensuring safety and reliability in hospitals and clinics. Medical professionals rely on each medical grade rechargeable battery to prevent interruptions in life-saving procedures. Battery failures, including lithium-ion battery pack outages, have caused significant patient harm and mortality. Medical grade rechargeable battery selection must meet strict safety and reliability standards, with rigorous testing and qualified manufacturers essential for patient safety. Lithium-ion chemistries dominate the market due to their reliability, safety, and high-performance characteristics. Testing, safety, and reliability must remain top priorities for every medical grade rechargeable battery powering modern medical devices.
Key Takeaways
Medical grade rechargeable batteries power critical medical devices with high safety and reliability, using advanced protections and strict testing to prevent failures that could harm patients.
Choosing batteries from experienced, certified manufacturers ensures compliance with international safety standards and supports device performance in diverse medical applications.
Lithium-ion batteries dominate medical use due to their long life, high energy, and safety features, making them ideal for devices like monitors, wheelchairs, and high-temperature equipment.
Part 1: Medical Grade Rechargeable Batteries
Definition
Medical grade rechargeable batteries represent a specialized category of power sources engineered for use in medical devices. These batteries deliver consistent performance, high reliability, and safety in critical healthcare environments. Manufacturers design medical grade rechargeable batteries to meet the demanding requirements of medical applications, including stable output voltage, long lifespan, and adaptability in size and shape. Unlike consumer-grade batteries, medical grade rechargeable batteries undergo rigorous testing and comply with strict regulatory standards. They feature advanced protection mechanisms such as short circuit protection, overcharge and over-discharge safeguards, over-current protection, and temperature control. These features ensure uninterrupted operation of medical devices, even in extreme or specialized environments.
Note: Medical grade rechargeable batteries must fit seamlessly into diverse medical device designs, from compact hearing aids to complex infusion pumps. Their adaptability supports innovation in medical technology.
Key Characteristics of Medical Grade Rechargeable Batteries
High reliability with stable output voltage and extended lifespan
Elevated energy density in a compact form factor
Robust performance in extreme or specialized environments
Customizable size and shape for specific medical device applications
Medical Device Risk Level | Battery Grade | Typical Medical Devices | Key Requirements |
|---|---|---|---|
Low-risk | Class I | Hearing aids, glucometers, blood pressure monitors | Low power consumption, lower risk |
Moderate-risk | Class II | Pacemakers, insulin pumps, nerve stimulators | Moderate power consumption, moderate risk |
High-risk | Class III | Implantable defibrillators, cochlear implants | High power consumption, long lifespan, high risk |
This classification demonstrates how medical grade rechargeable batteries are tailored to the risk level and power demands of each medical device.
Importance
Medical grade rechargeable batteries play a vital role in the reliability and safety of medical devices. The choice of battery technology directly impacts device performance, patient outcomes, and operational efficiency. Lithium-ion batteries dominate the market, powering over 60% of portable medical devices due to their superior energy density, lightweight design, and long lifespan. These attributes extend device operation and reduce replacement frequency, which is essential for high reliability in medical applications.
Medical professionals depend on medical grade rechargeable batteries to prevent power interruptions during critical procedures. The FDA has reported that up to 50% of system breakdowns in medical devices result from battery failures. Advanced diagnostic methods, such as Electrochemical Impedance Spectroscopy (EIS), help assess battery health and improve reliability. Without proper battery maintenance and testing, users may not recognize battery degradation, leading to unexpected failures.
Tip: Regular testing and monitoring of medical grade rechargeable batteries enhance device reliability and patient safety.
International Standards for Medical Grade Rechargeable Batteries
Standard | Scope and Relevance to Medical Grade Rechargeable Batteries |
|---|---|
IEC 62133 | Safety requirements for secondary cells and batteries with alkaline or non-acid electrolytes; includes biocompatibility and patient safety. |
UL 2054 | Safety standard for household and commercial batteries; covers electrical, mechanical, environmental, thermal safety, and performance. |
IEC 60601-1 | General requirements for basic safety and essential performance of medical electrical equipment, including batteries. |
ISO 13485 | Quality management system requirements for medical device manufacturers producing batteries. |
ISO 10993-1 | Biological safety evaluation of medical devices, ensuring batteries do not cause adverse biological reactions. |
UN 38.3 | Transportation safety standard for lithium-based batteries, ensuring safe shipping and handling. |
FDA & EU MDR | Regulatory mandates requiring compliance with above standards for safety, performance, labeling, and traceability of medical batteries. |
Compliance with these standards ensures that medical grade rechargeable batteries meet stringent safety, quality, and performance requirements. Manufacturers must conduct thorough testing to verify that each battery can withstand the demands of medical applications.
Medical grade rechargeable batteries support a wide range of medical applications, including infusion pumps, monitors, wheelchairs, and implantable devices. Lithium-ion batteries, with energy density up to 250 Wh/kg and durability over 500 charge cycles, provide the high reliability and performance stability required in these environments. Their widespread adoption highlights the importance of selecting the right battery technology for each medical device.
Part 2: Manufacturer Qualifications
Certifications
Manufacturers of medical grade rechargeable batteries must meet rigorous certification requirements to ensure safety, reliability, and regulatory compliance. In the United States, manufacturers follow ANSI standards such as ANSI C18.2M and ANSI C18.3M, which set safety requirements for rechargeable lithium battery packs. FDA compliance is essential, involving documentation, testing, and annual audits. In the European Union, CE marking is mandatory under the EU Battery Regulation (Regulation (EU) 2023/1542), which replaces Directive 2006/66/EC and applies to most batteries in medical devices. This regulation requires manufacturers to prepare technical documentation, carry out conformity assessments, and ensure batteries are designed for removability and replaceability. Global standards such as IEC 62133 and IEC 62281, along with UN38.3 for transport safety, provide a baseline for lithium-ion battery certification. Regional differences exist, with China requiring CCC certification and Japan mandating PSE certification. The table below compares key certifications and standards across major regions:
Region | Key Certifications and Standards | Regulatory Bodies / Notes |
|---|---|---|
United States | UL 1642, UL 2054, UN/DOT 38.3, ANSI C18.2M, ANSI C18.3M | UL, DOT, FDA, FCC, OSHA |
European Union | CE Marking, EN IEC 62485-5, EN IEC 62619, UN 38.3, EU Battery Regulation | EU regulatory framework, digital battery passport, traceability |
China | GB 31241-2014, GB/T 31485, CCC, UN 38.3 | Chinese national standards, CCC certification |
Japan | PSE Mark, METI safety guidelines | METI |
South Korea | KC Certification, KATS standards | Korean national certification system |
India | BIS standards (IS 16046, IEC 62133) | BIS |
Australia | ADR, AS/NZS battery safety standards | Australian regulatory framework |
Manufacturers must navigate both international standards and region-specific regulations governing lithium-ion battery certification to achieve market access and maintain compliance.
Experience
Leading manufacturers of medical grade rechargeable batteries demonstrate extensive experience in the industry. Large Power has produced custom lithium battery packs for over 23 years, advancing battery technology for medical applications. Experience correlates with improved quality control, effective testing, and reduced recall severity. A recent analysis of medical device recalls highlights that experienced manufacturers respond quickly to defects and communicate risks clearly, which helps protect patient safety. Manufacturers with deep expertise maintain strict compliance with regulations and standards, minimizing risks associated with lithium battery packs.
Supply Chain
The supply chain for medical grade rechargeable batteries presents unique challenges. Manufacturers must source biocompatible materials, design compact battery cases, and use advanced assembly technologies such as ultrasonic welding. This process bonds fragile electrodes and custom shapes without sparks or melting, ensuring battery integrity and compliance with medical standards. New ICAO regulations governing lithium-ion battery certification restrict shipping on passenger aircraft, requiring cargo-only transport and strict labeling. These regulations increase logistical complexity, oversight, and production delays. Healthcare providers may face availability issues due to longer lead times and supply disruptions. Manufacturers must audit suppliers, revise shipping routes, and manage inventory to maintain quality and compliance. Counterfeit and non-compliant batteries pose additional risks, making robust testing and supply chain management essential for reliable lithium battery packs.
Part 3: Types of Medical Batteries

Medical devices rely on advanced battery technologies to deliver reliable power, safety, and performance. The selection of battery chemistry directly affects device functionality, patient safety, and operational efficiency. The following overview highlights the most common battery types used in medical applications, with a strong focus on lithium-ion batteries and lithium battery packs.
Lithium-Ion
Lithium-ion batteries dominate the medical sector due to their high energy density, lightweight design, and long cycle life. Manufacturers customize medical lithium batteries to fit specific device requirements, including voltage, capacity, and form factor. Hospitals worldwide use lithium-ion batteries in infusion pumps, patient monitors, mobile X-ray units, and emergency equipment. These batteries offer up to five times longer lifetime than conventional batteries and maintain 80% capacity after 500 charge cycles. Advanced safety features, such as overcharge protection and thermal runaway mitigation, ensure compliance with medical standards like IEC60601 and UL. Medical lithium batteries also support digital communication for real-time status monitoring, enhancing reliability in critical care environments.
LiFePO4
LiFePO4 batteries provide enhanced safety and stability for medical devices. Their stable chemistry reduces the risk of thermal runaway, making them ideal for patient monitoring systems, infusion pumps, and medical implants. LiFePO4 batteries endure thousands of charge-discharge cycles, supporting extended device usage and reducing maintenance. Original Design Manufacturers tailor these batteries for specific medical applications, ensuring consistent power output and high performance. Their compact and lightweight design suits portable and wearable medical devices.
NiMH
NiMH batteries deliver reliable and consistent power for medical technology. Their safety profile and durability make them suitable for critical medical devices where performance cannot be compromised. NiMH batteries are commonly used in professional medical equipment, offering moderate cycle life and robust safety under physical or thermal stress. They require less complex battery management systems, which simplifies integration into medical devices.
NMC
NMC(Nickel, Manganese, Cobalt) combine high energy density with efficient charge and discharge rates. These batteries power compact, high-performance medical devices such as defibrillators and infusion pumps. Their cathode composition enhances energy storage and cycle performance, but they require advanced battery management systems due to lower thermal stability. Ternary cells support medical applications where space, weight, and reliable power are critical.
Medical lithium batteries, especially lithium-ion batteries, remain the preferred choice for modern medical devices due to their superior energy density, long lifespan, and advanced safety features.
Battery Type | Platform Voltage | Energy Density (Wh/kg) | Cycle Life (cycles) | Main Medical Applications |
|---|---|---|---|---|
Lithium-ion (LCO) | 3.7V | 150–200 | 500–1,000 | Monitors, infusion pumps, wearables |
LiFePO4 | 3.2V | 90–140 | 2,000–5,000 | Implants, monitors, pumps |
NMC (Ternary) | 3.7V | 232–293 | 800–1,000 | Defibrillators, compact devices |
NiMH | 1.2V | 60–120 | 500–1,000 | Professional medical equipment |
Medical lithium batteries continue to drive innovation and reliability in healthcare, supporting a wide range of devices and applications.
Part 4: Safety in Medical Grade Rechargeable Batteries
Medical grade rechargeable batteries must meet the highest safety requirements and testing procedures to ensure uninterrupted operation in critical healthcare environments. Safety and reliability remain the foundation of every lithium battery pack used in medical devices. Manufacturers integrate advanced protection features, conduct rigorous testing, and adhere to strict compliance protocols to minimize risks and meet global standards.
Protection Features
Manufacturers design medical grade rechargeable batteries with multiple layers of protection to address the unique demands of healthcare applications. These features prevent hazardous failures and enhance both safety and reliability. The most common protection features include:
Short circuit protection
Overcharge protection
Over-discharge protection
Over-current protection
Temperature protection
Equalization
These protection mechanisms play a vital role in reducing the risk of device failure. For example, thermal runaway can cause uncontrollable heating, leading to fires or explosions. Leaks of corrosive or toxic chemicals pose severe health hazards, while off-gassing releases combustible fumes that threaten patient and staff safety. Protection Circuit Modules (PCM) monitor and control cell voltages, stopping charging at safe limits and preventing overdischarge. PCMs also regulate maximum charge and discharge currents, disconnecting circuits if faults occur. Overdischarge protection is especially critical for implanted devices, where sudden power loss can have life-threatening consequences.
Smart battery systems often include advanced fuel gauges that track state-of-charge using coulomb counting, improving reliability. Cell balancing, both passive and active, corrects imbalances between cells, enhancing performance and safety. Temperature sensors monitor battery temperature to prevent overheating and optimize charging. Mechanical design features, such as robust enclosures and thermal management, protect against environmental and thermal damage. Internal short circuit mitigation strategies, enforced by PCMs and quality control, further reduce the risk of thermal runaway.
Tip: For more information on battery management systems (BMS) and protection circuit modules (PCM), consult Large Power.
Testing
Rigorous testing standards and regulations ensure that every medical grade rechargeable battery meets strict safety and reliability benchmarks before reaching the market. Testing protocols simulate real-world hazards and verify that batteries can withstand the stresses of daily use and transport.
UN 38.3 Testing
UN 38.3 is a mandatory transportation safety standard for lithium batteries, including medical grade rechargeable batteries. It requires a series of tests (T1-T8) to simulate hazards during transport:T1: Altitude Simulation (low pressure)
T2: Thermal Test (rapid temperature changes)
T3: Vibration
T4: Shock
T5: Short Circuit
T6: Impact (crush to cell case)
T7: Overcharge (for rechargeable batteries)
T8: Forced Discharge
Batteries are classified under UN 38.3 based on type and shipment method, such as UN 3480 for lithium-ion cells shipped alone and UN 3481 for batteries within devices. The standard also mandates design guidance, safety venting, prevention of external short circuits, and quality management during manufacturing. Packaging and labeling requirements help mitigate fire risks during transport. Manufacturers must maintain test reports and summaries for regulatory authorities.
IEC 62133 and Related Standards
Medical grade rechargeable batteries must comply with IEC 62133, which addresses safety, reliability, and regulatory compliance for medical devices. This standard covers risks such as overheating, leakage, and explosion. Compliance with IEC 62133 is often required alongside IEC 60601-1 for medical electrical equipment. UL standards like UL 1642 and UL 2054 may apply in certain markets, though they can limit international sales. These protocols ensure that batteries meet stringent safety requirements and testing procedures before market approval.Additional Testing Protocols
Manufacturers also conduct internal short circuit, overcharge, and forced discharge tests to verify battery safety. Advanced diagnostic methods, such as Electrochemical Impedance Spectroscopy (EIS), assess battery health and improve reliability. Testing standards and regulations require manufacturers to document all procedures and maintain traceability throughout the supply chain.
Compliance
Compliance with regulations and certification standards is essential for market access and patient safety. Regulatory bodies worldwide oversee the safety and reliability of medical grade rechargeable batteries, ensuring that manufacturers meet all safety requirements and testing procedures.
Regulatory Body / Organization | Role / Scope of Oversight |
|---|---|
U.S. Food and Drug Administration (FDA) | Regulates medical devices including batteries; requires compliance with recognized consensus standards for safety and performance before market entry. |
Federal Aviation Administration (FAA) | Regulates batteries used in aviation-related medical devices; enforces standards like TSO-C142a and RTCA DO-227 for safe operation on airplanes. |
International Civil Aviation Organization (ICAO) | Oversees international air transport regulations for lithium battery safety during shipping. |
International Air Transport Association (IATA) | Provides guidelines and regulations for safe air transport of lithium batteries. |
International Maritime Organization (IMO) | Regulates maritime transport safety for lithium batteries. |
United States Department of Transportation (DOT) | Regulates transportation safety including lithium battery shipping requirements. |
ANSI/AAMI | Develops standards for medical electrical equipment safety and performance, including battery requirements (e.g., ANSI/AAMI ES 60601-1). |
IEC (International Electrotechnical Commission) | Provides battery safety standards such as IEC 60086-4 and IEC 62133 for primary and secondary lithium batteries. |
ISO (International Organization for Standardization) | Issues standards related to medical devices and batteries in specific fields (e.g., dentistry, ophthalmic instruments). |
UL (Underwriters Laboratories) | Provides safety standards for lithium and household batteries (e.g., UL 1642, UL 2054). |
CSA Group | Certification and testing body assisting manufacturers in compliance with international regulations and UN transportation requirements (UN 38.3). |
The FDA regulates medical device batteries in the United States, requiring manufacturers to demonstrate compliance with recognized safety and performance standards as part of premarket submissions. International standards such as ISO, IEC, UL, and UN transportation regulations also apply. Collaboration among manufacturers, medical device companies, and regulatory bodies ensures that batteries meet the highest safety and reliability standards.
Non-compliance with regulations can result in severe penalties. These include shipment rejection, legal consequences, lawsuits, reputational damage, increased health and safety risks, and even fatal accidents. The table below outlines baseline penalties for lithium battery shipping violations under US DOT Hazardous Materials Regulations (HMR):
Violation Type | Baseline Penalty Amount |
|---|---|
Offering lithium batteries not protected against short circuit | $15,000 |
Offering lithium batteries in unauthorized packages | $12,500 |
Offering lithium batteries on passenger aircraft or misclassifying for air transport | $30,000 |
Failure to prepare batteries to prevent damage in transit | $6,000 |
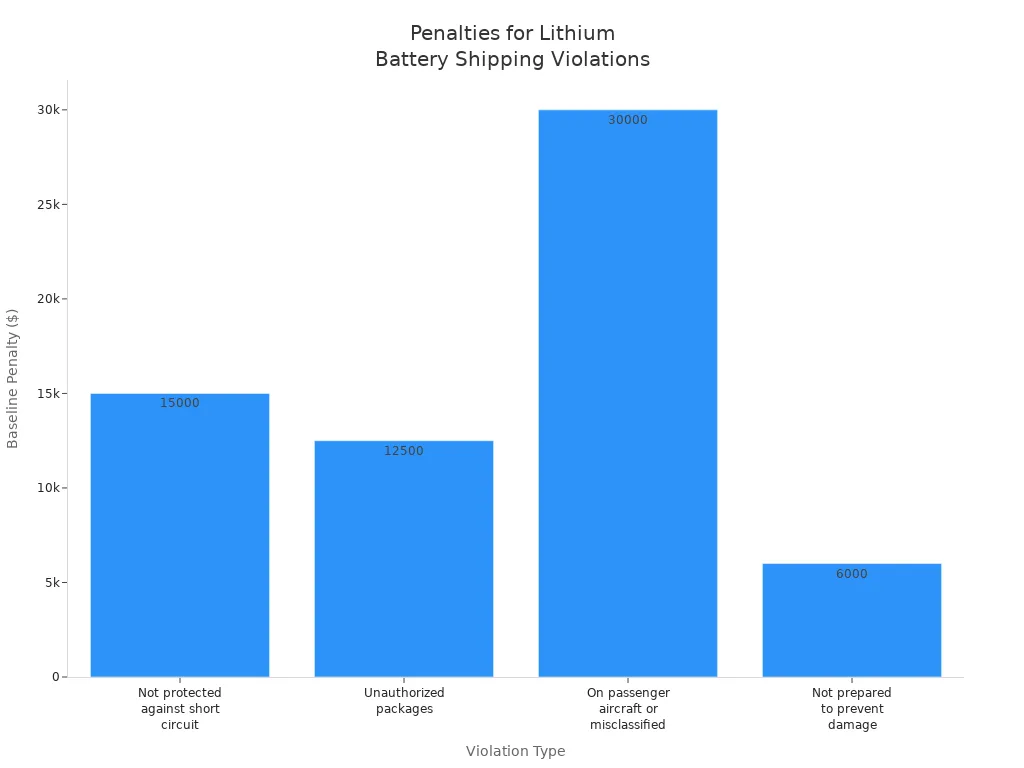
Compliance with regulations and certification standards protects both patients and healthcare providers. It ensures that every lithium battery pack meets the highest safety and reliability benchmarks, supporting the critical role of medical devices in patient care.
Part 5: Medical Applications and Case Studies

Wheelchairs
Medical wheelchairs rely on advanced battery technologies to deliver consistent power and safety. Lithium-ion batteries have become the preferred choice for these medical applications due to their high energy density, lightweight design, and long lifespan. Compared to traditional lead-acid or AGM batteries, lithium-ion batteries offer up to 4,000 charge cycles, fast charging within 1–2 hours, and virtually maintenance-free operation. These features improve the reliability of portable medical equipment and reduce downtime for users. The table below compares common battery types used in electric wheelchairs:
Battery Type | Voltage | Capacity (Ah) | Lifespan | Weight | Maintenance | Charging Time |
|---|---|---|---|---|---|---|
AGM | 12/24V | 20–80 | 1–3 years | Heavier | Low, sealed | Moderate |
Gel | 12/24V | 20–80 | 1–3 years | Moderate | Maintenance-free | Slow |
Lithium-ion | 12/24V | Variable | Up to 5 years | Maintenance-free | Fast (1–2 hr) |
Lithium-ion batteries also meet airline safety standards, making them suitable for travel-related medical device battery examples.
Monitors
Medical monitors require batteries that provide stable power and advanced safety features. Lithium-ion batteries dominate these applications because they support real-time monitoring and rapid response to abnormal conditions. Integrated sensors detect overheating, pressure changes, and strain, which helps prevent thermal runaway and other hazards. Battery specifications guide initial selection, but rigorous testing and continuous monitoring ensure that batteries meet the strict operational and safety requirements of medical monitors. Manufacturers often use custom lithium-ion battery packs to optimize performance in specific applications, such as patient monitoring and wearable drug delivery systems.
Note: Advanced testing and supplier evaluation remain essential for reliable battery performance in medical monitors.
High-Temperature Devices
Specific applications include:
Medical carts and robots
Surgical tools
Security and infrastructure monitoring systems
Lithium-ion batteries continue to set the standard for safety, performance, and versatility in a wide range of medical and industrial applications.
Reliable batteries protect patient safety.
Qualified suppliers ensure safety and compliance.
FAQ
What distinguishes medical grade lithium-ion battery packs from consumer-grade batteries?
Medical grade lithium-ion battery packs feature advanced safety mechanisms, rigorous testing, and compliance with standards like IEC 62133. These packs deliver reliable performance in critical healthcare environments.
How often should medical lithium battery packs undergo safety testing?
Manufacturers recommend annual safety testing for medical lithium battery packs. Regular diagnostics help maintain reliability and ensure compliance with regulatory standards.
Which lithium battery chemistry offers the longest cycle life for medical devices?
LiFePO4 lithium battery packs provide the longest cycle life, supporting up to 5,000 cycles. These packs suit applications requiring extended durability and high safety.




