You often see medical applications choosing between primary and Rechargeable Batteries based on reliability, energy density, and how each device works. Medical grade rechargeable batteries, especially those using lithium chemistries like LiFePO4 and NMC, deliver high energy density and a long operational life. Manufacturers focus on factors such as cost, patient compliance, and new safety features.
Recent innovations in lithium battery packs improve efficiency and safety, making them ideal for many medical applications.
Key Takeaways
Choose rechargeable batteries for devices needing frequent use. They offer high energy density and lower long-term costs.
Select primary batteries for implantable devices. They provide long shelf life and reliability without the need for recharging.
Consider the environmental impact of battery choices. Rechargeable batteries reduce waste over time, while primary batteries create more disposal issues.
Evaluate total cost of ownership, not just initial price. Rechargeable batteries may cost more upfront but save money in the long run.
Ensure compliance with safety standards. Using batteries that meet regulations enhances device safety and reliability.
Part 1: Battery Differences

1.1 Rechargeable Batteries
You rely on rechargeable batteries for medical devices that demand frequent use and consistent performance. These batteries use reversible electrochemical reactions, which allow you to recharge them many times. In medical applications, lithium-ion chemistries such as LiFePO4, NMC, LCO, LMO, and LTO stand out for their high energy density and long cycle life. You find LiFePO4 batteries in portable medical devices and surgical tools because they deliver stable voltage and safety. Nickel Metal Hydride and Nickel Cadmium batteries also serve in small devices like blood pressure monitors and diabetic monitors.
Tip: You should choose rechargeable batteries when your device needs high current draw and repeated use. These batteries reduce waste and lower long-term costs.
Rechargeable batteries offer higher power output, making them suitable for equipment that requires reliable and strong energy delivery. You benefit from their ability to support lithium battery packs, which improve efficiency and safety in medical environments. Although rechargeable batteries cost more upfront, you save money over time due to their reusable nature. Their high energy density means you can design compact devices without sacrificing performance.
Battery Type | Rechargeable | Applications |
|---|---|---|
LiFePO4 | Yes | Portable medical devices, surgical tools |
NMC | Yes | Advanced medical equipment |
LCO | Yes | Diagnostic imaging devices |
LMO | Yes | Infusion pumps, monitoring systems |
LTO | Yes | Rapid charge/discharge medical devices |
Nickel Metal Hydride | Yes | Small medical devices |
Nickel Cadmium | Yes | BP monitors, diabetic monitors |
1.2 Primary Batteries
You use primary batteries in medical devices that require long shelf life and reliability without the need for recharging. These batteries rely on non-reversible electrochemical reactions, which means you dispose of them after a single use. Primary batteries, such as LiMnO2 and lithium-carbon fluorides (CFx), power critical devices like defibrillators and cardiac pacemakers. Alkaline and zinc air batteries also play a role in blood pressure monitors and pulse oximeters.
Primary batteries provide a longer shelf life due to low self-discharge rates. You choose them for devices where replacement is difficult or impossible, such as implantable medical devices. While primary batteries offer lower power output compared to rechargeable batteries, they excel in applications that demand stability and reliability over extended periods. Their high energy density supports compact device design, but you must consider the environmental impact due to increased waste.
Feature | Primary Batteries | Rechargeable Batteries |
|---|---|---|
Rechargeability | Not rechargeable | Rechargeable |
Electrochemical Reaction | Non-reversible | Reversible |
Cost | Generally cheaper | Typically more expensive |
Shelf Life | Longer due to low self-discharge | Shorter compared to primary batteries |
Power Output | Lower power output | Higher power output, suitable for high current draw applications |
Environmental Impact | More waste per battery | Less waste after multiple recharge cycles |
You must evaluate the specific needs of your medical device before selecting the right battery. Consider the importance of high energy density, reliability, and whether the device benefits from single-use or reusable batteries.
Part 2: Performance Comparison
2.1 Longevity & Replacement
You need to consider battery longevity and replacement cycles when choosing batteries for medical devices. Rechargeable batteries, especially lithium battery packs using LiFePO4, NMC, LCO, LMO, and LTO chemistries, offer a long service life. These batteries can last up to 25 years in some applications. Primary batteries, such as those used in pacemakers, typically last 5 to 10 years before replacement becomes necessary.
Battery Type | Typical Lifespan |
|---|---|
Rechargeable | Up to 25 years |
Primary (e.g., Pacemakers) | 5 to 10 years |
You benefit from fewer replacements when you use rechargeable batteries. This reduces maintenance costs and minimizes device downtime. Lithium battery packs also support high cycle life, with many models retaining 80% of their capacity after 500 charge-discharge cycles. This reliability is crucial for medical devices that require consistent performance over many years.
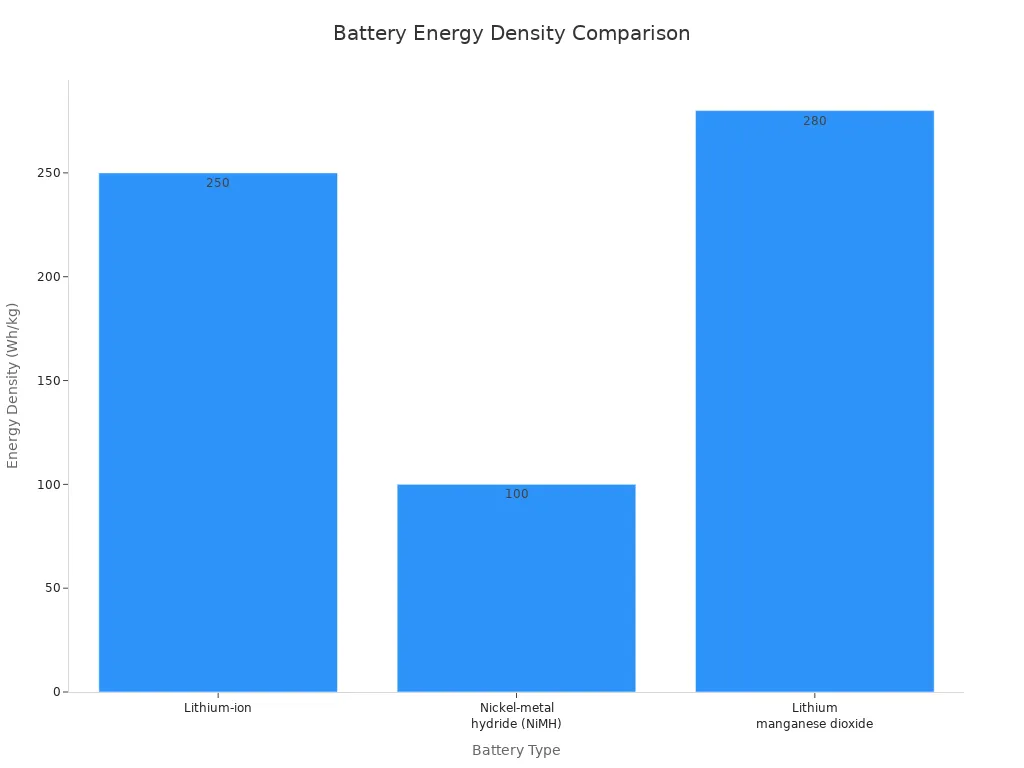
2.2 Energy Density & Size
Energy density and size play a major role in medical device design. You want batteries that deliver high energy in a compact form. Lithium-ion batteries, including LiFePO4, NMC, LCO, LMO, and LTO, provide energy densities up to 250 Wh/kg. Primary lithium manganese dioxide batteries reach about 280 Wh/kg, making them suitable for devices where space is limited.
Battery Type | Energy Density (Wh/kg) |
|---|---|
Lithium-ion | Up to 250 |
Nickel-metal hydride (NiMH) | Around 100 |
Lithium manganese dioxide | About 280 |
You gain several advantages with lithium battery packs:
High energy density allows longer operation between charges.
Lightweight design improves portability for handheld and wearable devices.
Long service life reduces the need for frequent replacements.
You can design smaller, lighter medical devices without sacrificing reliability or performance. This is especially important for portable and wearable medical equipment.
2.3 Reliability & Safety
Reliability and safety are top priorities in medical applications. You depend on batteries that deliver stable performance and minimize risks. Medical grade rechargeable batteries must meet strict safety engineering standards. These include compliance with IEC 62133, UL 2054, ISO 13485, and IEC 60601-1. You also require biocompatibility, overcharge protection, thermal shutdown, battery management systems, authentication, and serialization for traceability.
Safety Feature | Description |
|---|---|
Compliance Standards | Batteries must meet IEC 62133, UL 2054, ISO 13485, and IEC 60601-1. |
Biocompatibility | Batteries must be biocompatible to ensure safety near patients. |
Safety Features | Must include overcharge protection, thermal shutdown, and battery management systems. |
Authentication | Batteries must be authenticated to prevent counterfeiting. |
Serialization | Batteries must be serialized and traceable for safety monitoring. |
You rely on lithium battery packs for their advanced safety features and consistent reliability. These batteries help you avoid unexpected failures and ensure patient safety.
Environmental Impact & Cost-Effectiveness
You must also evaluate the environmental impact and cost-effectiveness of batteries. Rechargeable batteries use more toxic materials, but they reduce resource depletion and manufacturing impact when used to their full potential and recycled. Primary batteries create more waste and have a higher overall environmental impact due to frequent disposal.
Impact | Rechargeable Batteries | Disposable Batteries |
|---|---|---|
Raw Materials Used | More toxic materials | Less toxic materials |
Resource Depletion | Lower | Higher |
Manufacturing Impact | Lower | Higher |
Use Impact | Lower if charged ~50 times | Higher |
Energy Consumption | Higher | Lower |
Pollution | Mining-related | Disposal-related |
Disposal Impact | Higher if not recycled | Lower |
Overall Impact | Lower if used to full potential and recycled | Higher |
You can learn more about sustainability here and about conflict minerals here.
Note: You should always consider the full lifecycle of batteries to maximize cost-effectiveness and minimize environmental harm.
Part 3: Cost & Maintenance
3.1 Upfront vs. Long-Term Cost
You face important decisions when comparing the cost of batteries for implantable biomedical devices. Primary batteries often attract you with a lower initial cost. You can install primary cell batteries in implantable devices without a large upfront investment. However, you must consider the long-term expenses. Secondary batteries, such as lithium-ion chemistries like LiFePO4, NMC, LCO, LMO, and LTO, require a higher initial investment. You benefit from their ability to recharge and reuse them hundreds or thousands of times.
Here is a table that compares cost factors for primary batteries and secondary cell batteries in implantable biomedical devices:
Aspect | Rechargeable Batteries | Primary Batteries |
|---|---|---|
Initial Cost | Higher initial investment | Lower initial cost |
Longevity | Can be reused hundreds to thousands of times | Single-use, needs frequent replacement |
Replacement Frequency | Eventually needs replacement, but less often | Frequent replacement required |
Maintenance Costs | Proper care can extend life, reducing costs | No maintenance, but higher overall costs |
Environmental Impact | Less waste, fewer hidden costs | More waste, hidden costs from disposal |
You save money over time with secondary batteries in implantable biomedical devices. You reduce replacement frequency and lower hidden costs related to waste disposal. Primary cell batteries may seem cheaper at first, but you pay more in the long run due to frequent replacements.
Tip: You should always calculate total cost of ownership, not just the initial price, when choosing batteries for implantable devices.
3.2 Maintenance Needs
You must understand the maintenance requirements for batteries in implantable biomedical devices. Secondary batteries, especially lithium battery packs, need regular attention. You should follow safety standards such as ANSI/AAMI ES 60601-1 and IEC guidelines. These standards help you ensure safe operation of implantable devices powered by secondary cell batteries.
Routine maintenance for secondary batteries includes:
Observe and note the run time of a new fully-charged battery to compare with older batteries.
Routinely check the battery’s charge status.
Monitor batteries approaching the end of their estimated life.
Replace if run time drops below 80% of original or charge time increases significantly.
Charge to 50% before storage and check every six months.
Avoid disassembly, crushing, or exposure to extreme temperatures.
You should design implantable biomedical devices to use only approved replacement batteries and chargers. You must include strong language in Instructions for Use to outline approved storage, charging, use, and maintenance requirements.
Primary batteries in implantable devices require less maintenance. You do not need to recharge them. You can easily replace primary cell batteries, and they offer a longer shelf life. However, you must dispose of them after use, which increases environmental impact.
Here is a table comparing maintenance needs for primary batteries and secondary batteries in implantable biomedical devices:
Battery Type | Maintenance Needs | Advantages | Disadvantages |
|---|---|---|---|
Primary (Disposable) | No need for recharging; easy to replace; longer shelf life | Convenient; readily available; ideal for devices needing frequent changes | Environmental impact due to waste; cannot be recharged; must be disposed of after use |
Rechargeable | Requires regular recharging; may have a higher self-discharge rate | Long-term cost savings; eco-friendly; reusable multiple times | Shorter lifespan than disposables; may be inconvenient for users needing constant power availability |
You must weigh the convenience of primary batteries against the long-term savings and sustainability of secondary batteries. You should choose the battery type that best fits your implantable biomedical device’s needs.
Part 4: Applications

4.1 Implantable Biomedical Devices
You depend on implantable biomedical devices for life-saving functions. Pacemakers, neurostimulators, and drug delivery systems require a reliable power source that can operate for years without maintenance. Most implantable biomedical devices use primary batteries. These batteries utilize lithium metal anodes with advanced cathode systems, which provide long service life and stable output. You see primary batteries in pacemakers because they offer high energy density and low self-discharge rates. This means you do not need to recharge or replace them frequently.
Battery Type | Description |
|---|---|
Primary Batteries | Utilize lithium metal anodes with various cathode systems, providing long service life. |
Rechargeable Batteries | Secondary lithium-ion batteries that can be charged while implanted, but generally have lower capacity and shorter lifespan. |
You notice that the first pacemaker in 1958 used a rechargeable nickel-cadmium battery. Early biomedical devices relied on mercury-zinc batteries, but safety concerns led to their discontinuation. The introduction of lithium-iodine batteries in 1975 improved lifespan and reliability. Today, you choose primary batteries for most implantable biomedical devices because they minimize the need for surgical replacement and maximize patient safety.
Tip: You should select primary batteries for implantable biomedical devices when you need long shelf life and minimal maintenance.
4.2 Portable Devices
You use portable biomedical devices every day in hospitals, clinics, and home care settings. Devices such as infusion pumps, insulin pumps, electrocardiogram monitors, and patch-based ECG systems require batteries that deliver consistent power and support frequent use. Rechargeable batteries dominate this space. You rely on lithium-ion chemistries like LiFePO4, NMC, LCO, LMO, and LTO for their high energy density, long cycle life, and fast charging capabilities.
Battery Type | Advantages |
|---|---|
Lithium-Ion | High energy density, long-lasting battery life, fast charging, low self-discharge rates. |
Nickel-Cadmium | Excellent durability and reliability, long cycle life, withstands high discharge rates. |
Nickel-Metal Hydride | Good balance of energy density and safety, more compact and lightweight than NiCd batteries. |
You find lithium battery packs in portable biomedical devices because they support high current draw and repeated recharge cycles. You use 18650 lithium batteries for their high energy storage and consistency. Polymer lithium batteries offer customizable shapes and improved safety performance. These batteries reduce explosion risks and integrate seamlessly into patch-based ECG monitors and wearable biomedical devices.
Battery Type | Key Features | Contribution to Safety and Performance |
|---|---|---|
18650 Lithium | High energy density, good consistency | Enhances energy storage and reliability in portable devices. |
Polymer Lithium | Customizable shapes, improved safety performance | Reduces risk of explosion and allows for better integration. |
18650 batteries provide good consistency and high energy storage.
Polymer batteries offer better safety performance and can be customized to fit various biomedical device designs.
Unlike liquid batteries, polymer batteries only bulge in case of an accident, reducing explosion risks.
You also see lithium battery packs used in robotics, security systems, infrastructure, consumer electronics, and industrial sectors. Standardized lithium battery chemistries such as LiFePO4, NMC, LCO, LMO, and LTO ensure consistent platform voltage, energy density, and cycle life across these applications.
Note: You should always use battery management systems (BMS) to monitor battery health and prevent failures.
4.3 Emergency & Disposable Use
You rely on emergency and disposable biomedical devices for critical situations. Devices such as flashlights, radios, and patch-based ECG monitors need batteries with long shelf life and instant readiness. You choose primary batteries for these applications because they provide reliable power source even after long periods of storage. You use disposable batteries in travel kits, outdoor activities, and emergency medical kits where charging options are limited.
Infrequently used devices, such as flashlights and radios, where long shelf life is advantageous.
Travel and outdoor activities, where charging options may be limited.
Emergency kits, providing reliable power when needed most.
When you use disposable batteries, you must follow safety tips. Never mix old and new batteries. Store batteries in a cool, dry place. Dispose of batteries properly to prevent environmental harm.
Key Considerations | Description |
|---|---|
Energy Density | Lithium-ion batteries outperform alkaline batteries, providing more energy in a smaller size. |
Cycle Life | They offer significantly longer cycle life, reducing the frequency of replacements. |
Safety Certifications | Improved safety certifications ensure protection against hazards in medical settings. |
Performance Metrics | Enhanced performance with more lift cycles per charge and faster recharge times. |
Environmental Impact | Greener technology that eliminates lead acid disposal issues. |
Extended device lifespan leads to fewer replacements and less waste.
Lower frequency of battery changes minimizes downtime during emergencies.
Seamless integration with biomedical equipment improves operational efficiency.
Smart monitoring technology gives you real-time data on battery health and state of charge. This ensures emergency readiness and improves patient outcomes. Advanced Battery Management Systems (BMS) provide insights that help you prevent unexpected failures and maintain operational excellence.
You see lithium battery packs used in patch-based ECG monitors, emergency biomedical devices, and portable medical equipment. These batteries deliver reliable power source and support advanced features such as smart monitoring and fast recharge. You benefit from standardized lithium battery chemistries, which ensure safety and performance across medical, robotics, security, infrastructure, consumer electronics, and industrial sectors.
Part 5: Standards & Compliance
5.1 Regulatory Needs
You must follow strict regulations when you select batteries for medical devices. In Europe, the EU Battery Regulation (Regulation (EU) 2023/1542) sets requirements for sustainability, safety, labeling, and information. This regulation covers all categories of batteries used in medical devices except for implanted and infectious products. You need to provide a carbon footprint statement, ensure removability and replaceability of device batteries, and meet labeling and information requirements. Economic operators must fulfill specific obligations to keep batteries compliant.
The EU Battery Regulation focuses on sustainability and safety.
You must label batteries clearly and provide information for traceability.
Removability and replaceability help you maintain devices efficiently.
In the United States and globally, you must comply with standards for both primary and rechargeable batteries. The table below shows key standards:
Battery Type | Applicable Standards | Description |
|---|---|---|
Primary Batteries | IEC 60086-4 | Safety of lithium batteries, ensuring safe operation under intended use and foreseeable misuse. |
Rechargeable Batteries | IEC 62133 | Requirements for safe operation of portable sealed secondary lithium cells and batteries containing non-acid electrolyte under intended use and foreseeable misuse. |
You should always use lithium battery packs that meet these standards. Consistent platform voltage, energy density, and cycle life are essential for medical grade lithium chemistries like LiFePO4, NMC, LCO, LMO, and LTO. For more details on sustainability and conflict minerals, review our sustainability statement and conflict minerals statement.
Tip: You improve device safety and reliability by choosing batteries that meet regulatory standards.
5.2 Risk Management
You must manage risks when you design and use batteries in medical devices. Risk management strategies help you prevent failures and ensure patient safety. You need to select the right battery technology, assess risks, and verify design through testing. Abuse testing and advanced analyses, such as MRI compatibility and sterilization risk assessments, are vital steps. Failure analysis lets you identify root causes and apply corrective actions.
Strategy Type | Description |
|---|---|
Battery Selection | Choose battery technology based on performance and safety criteria. |
Evaluate potential risks associated with battery use in medical devices. | |
Regulatory Compliance | Ensure adherence to relevant regulations and standards for battery safety in medical applications. |
Design Verification and Validation | |
Abuse Testing | Simulate extreme conditions to assess battery resilience and safety. |
Advanced Analyses | Conduct specialized tests like MRI compatibility and sterilization risk assessments. |
Failure Analysis | Investigate battery failures to identify root causes and implement corrective actions. |
You should use risk management methods such as Failure Mode and Effects Analysis (FMEA) and system architecture planning. Manufacturers assess market, technology, regulatory, and safety risks. You must prototype and test batteries to validate components. Risk-based maintenance (RCM) started in the airline industry and now supports reliability in healthcare. You increase reliability and safety by applying these strategies to lithium battery packs and other batteries in medical devices.
Note: You protect patients and reduce costs by following risk management best practices for batteries.
You should match battery type to your medical device’s needs. Use primary batteries for implantable devices that require long shelf life and minimal maintenance. Choose rechargeable lithium battery packs (LiFePO4, NMC, LCO, LMO, LTO) for portable equipment that demands frequent use and high power.
Quick B2B Decision Guide:
Prioritize reliability and safety certifications.
Factor in shelf life, temperature tolerance, and pulse requirements.
Always test batteries under real conditions, train staff, and follow regulatory standards. Consider sustainability, labeling, and end-of-life recycling to meet compliance and environmental goals.
Aspect | Details |
|---|---|
Quality Assurance | Maintain consistent battery performance. |
Sustainability | Use recycled materials and eco-design principles. |
End-of-Life Management | Establish recycling and collection schemes for used batteries. |
FAQ
What lithium battery chemistry should you choose for portable medical devices?
You should select LiFePO4 or NMC lithium battery packs. These chemistries offer high energy density, long cycle life, and stable platform voltage. They support frequent charging and deliver reliable performance in portable medical equipment.
How do you ensure safety when using lithium battery packs in medical devices?
You must use batteries that meet IEC 62133 and UL 2054 standards. Always integrate battery management systems (BMS) for overcharge protection, thermal shutdown, and traceability. This reduces risks and improves patient safety.
Why do implantable medical devices often use primary batteries instead of rechargeable lithium packs?
You rely on primary batteries for implantable devices because they provide long shelf life and stable output. Rechargeable lithium packs have shorter lifespans and may require maintenance, which is not practical for implants.
What maintenance steps should you follow for lithium battery packs in medical applications?
You should monitor charge status, replace batteries when capacity drops below 80%, and store packs at 50% charge. Avoid extreme temperatures and use only approved chargers. Routine checks help maintain safety and performance.
How does energy density affect your choice of lithium battery pack for medical devices?
Higher energy density lets you design smaller, lighter devices. Lithium chemistries like LCO and LMO offer up to 250 Wh/kg, supporting compact medical equipment without sacrificing power or reliability.




